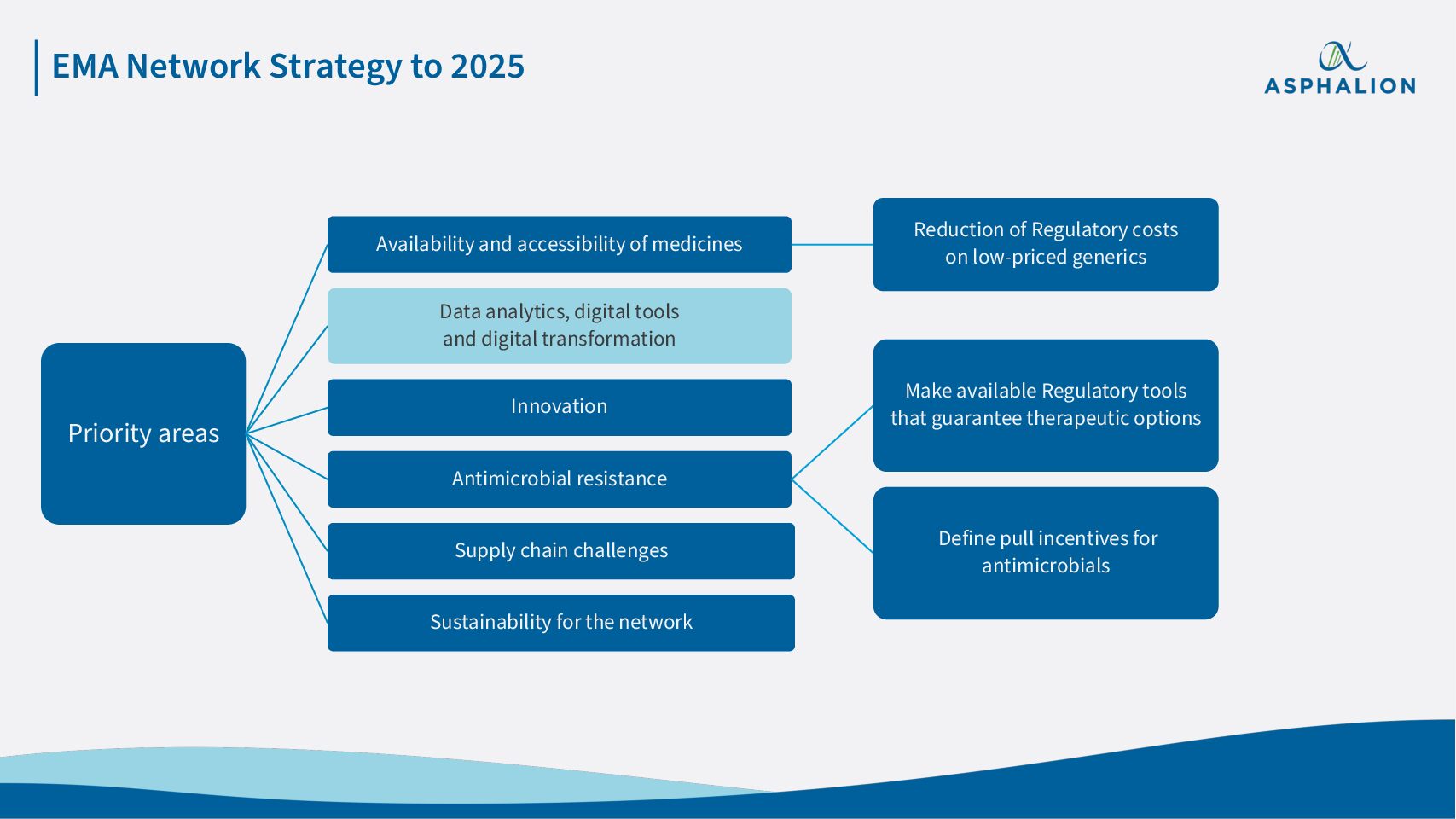
The European Medicines Agency Network Strategy to 2025 was detailed just last week during the 20th Regulatory Affairs and Pharmacovigilance conference.
The strategy sets out how the network will continue to enable the supply of safe and effective medicines, in the face of developments in science, medicine, digital technologies, globalisation and emerging health threats, such as the Covid-19 pandemic.
One of the major priority areas is Digitalisation, a subject that is being widely discussed as for how it is meant to be implemented within the frame of the Pharmaceutical Strategy for Europe.
Our Asphalion experts Lidia Cánovas, General Manager, Regulatory Affairs and Eduardo Garrido, Medical Device Officer are attending the congress. Next congress sessions are scheduled for the 27th and 29th of January and the 2nd and 4th of February.
At Asphalion, we are excited to see how all the situation evolves! We will keep you posted!
If you have any questions do not hesitate to contact us at: [email protected]