Regulatory, Scientific & Safety Consulting in Life Sciences
Barcelona | Madrid | Pamplona | Munich | London

We are a one-stop shop, able to offer you all solutions for all kind of projects, with a team that is constantly striving for excellence, always oriented to providing you with the best results
Mission
Our team’s mission is our passion: Working together with our clients in order to generate ideas and implement solutions that contribute to improving health in all our lives.
Vision
Our dream is to be a global reference and a driving force in the evolution of the healthcare sector, offering the best environment for the individual and collective growth of our team and clients.
Values
- Passion
- Flexibility
- Commitment
- Quality
- Companionship
Mission
Our team’s mission is our passion: Working together with our clients in order to generate ideas and implement solutions that contribute to improving health in all our lives.
Vision
Our dream is to be a global reference and a driving force in the evolution of the healthcare sector, offering the best environment for the individual and collective growth of our team and clients.
Values
- Passion
- Flexibility
- Commitment
- Quality
- Companionship
A bit of history...
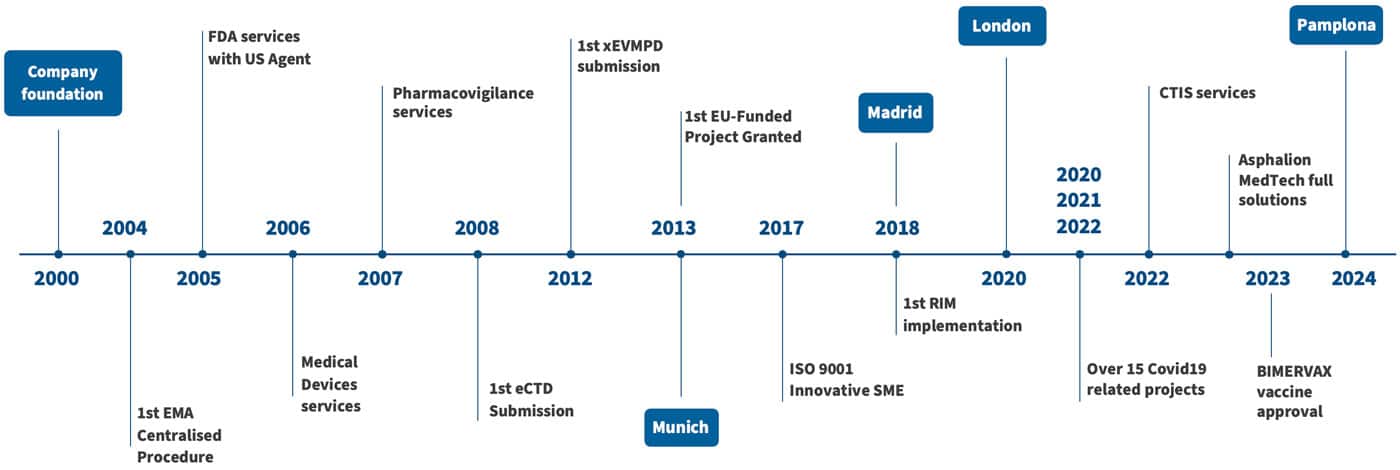
Since its founding in 2000 by a small group of pharmaceutical R&D professionals with the vision to offer high-quality services for the development and registration of innovative drugs, Asphalion has evolved into a leading consultancy firm in regulatory affairs, life sciences services, marked by innovation and regulatory excellence.
Within just a few years, Asphalion contributed to global development and registration projects and successfully participated in a European Centralised Procedure and US New Drug Application.
Along with early success stories, Asphalion’s team and clientele expanded rapidly, leading to the establishment of specific teams per service area, which eventually evolved into Asphalion’s eight service units of today.
In 2008, Asphalion identified the internationalization of both its service capabilities and customer base as a strategic necessity for long-term company growth. Taking decisive actions, Asphalion built a network of worldwide partners, increased the number of international staff, and directed its commercial efforts towards new markets. This effort resulted in a balanced revenue stream, with approximately 50% coming from local clients in Spain and 50% from international clients.
Embracing technological advancements, the company pioneered electronic submissions and adapted swiftly to evolving regulatory requirements. Nowadays, Asphalion continues at the cutting edge of tech-driven innovation in all areas related to life sciences regulation.
Amidst the COVID-19 pandemic, in line with its vision of “implementing solutions that contribute to improving the health of everyone’s lives”, Asphalion worked hard in numerous COVID projects. Of particular importance was Asphalion’s agility and resilience in securing approval for the BIVERMAX® vaccine in 2023.
After years of sustained organic growth, Asphalion has emerged as a leading consultancy with over 180 team members, serving a clientele of over 1,000 companies and boasting a track record of over 5,000 projects. The company offers comprehensive support for all regulatory, scientific, and safety activities throughout the development, registration, and commercialization of medicines and medical devices.
Its strong track record has made ASPHALION a reference in the international healthcare sector and a key stakeholder in the implementation of new regulatory standards
ASPHALION’s experts have delivered solutions to over 1,000 Pharmaceutical, Biotechnological and Medical Technology companies from more than 50+ countries in over 5,000 projects covering non-clinical and clinical development, CMC, dossier writing, regulatory procedures, vigilance, eSubmissions and data management for both medicinal products and medical devices.




ASPHALION offers expert consulting, strategic advice, operational support and full outsourcing services for all drug development stages
To deliver the best possible solution in each project, ASPHALION works closely with international competent authorities, notified bodies, scientific associations and key opinion leaders and maintains a comprehensive network of geographic and functional partners.
Corporate social responsibility
Key success factors
Our key to success is that we put ourselves in our customer’s shoes, and that all customers are equally important to us.
For our team, the project is the core of everything: easy or complex; from a small or a big company. Saving time in registering a medicine does not only mean the commercialization can be reached faster, but it also means increasing its life and exclusivity.
In other words, the customer ends up saving more money than the cost of our services, and this is Asphalion’s open secret of success.
Quality
Used to delivering results in complex situations and high satisfaction rate from our clients.
Flexibility
Constantly adapting to different requirements and working with tight schedules.
Commitment
Passionate and qualified team willing and dedicated to every project, always up-to-date to the latest standards.
Expertise
Our expertise, which is rooted in seniority and engagement, transcends all expectations.



















