During the first steps of clinical development stage, preliminary data consolidation and aggregation become fundamental to ensure an appropriate safety and efficacy profiling that best validates a pharmaceutical to proceed. Particularly for early clinical phases (Phase I and II) transferring all generated pre-clinical information to the clinical setting represents a big challenge.
Additionally, development requires to escalate the conclusions to larger, representative populations. These late phases of development are especially subjected to a highly complex regulatory context as the development approaches potential product registration and commercialization.
In order to guarantee success, the exact scope, timing and depth of clinical development plan must be strategically analyzed involving local regulatory authorities and stakeholders to ensure enough evidence generation according to regulatory requirements.
All this activity generates a strategic workflow of contacts, specific meetings as well as data and documents that must be planned and managed in terms of time and resources to ensure this efficient clinical execution that best allows to proceed successfully to further phases.
Asphalion has a broad experience in this matter with regards to:
Clinical development stage Solutions
1) Regulatory roadmap oriented towards future product commercialization:
2) Strategic consultancy throughout the clinical development plan.
3) Regulatory Tools
4) Support in Scientific Advice meetings with regulatory authorities, including strategic and operative support in the meeting. Preparation of Briefing Package, attendance to meetings with authorities and adequation of the meeting content to comply with company plans.
5) Medical writing services for clinical and non-clinical evidence generation, including:
6) Support in clinical trial applications and submissions in EU/FDA:
7) Paediatric Investigational Plan (PIP)
8) Management of Orphan Drug Designation (ODD) procedure in EU and US:
9) Pre-approval development support in UK market application and entity requirements:
Schedule a free meeting!
Discuss your case with our experts and receive a valuable feedback
Share
Why Asphalion?
Asphalion knows and can handle with experience the different intermediaries, stakeholders, regulations, necessary documents and tools that are needed throughout the early phase of clinical development in order to maximize the transfer of pre-clinical research to a clinical setting in a strategic manner that best responds to their client’s commercialization objectives.
Asphalion has more than 20 years of experience both in administrative support and management in clinical trial applications and maintenance, as well as in medical writing activities and regulatory strategy focused on the generation of clinical evidence oriented to comply with requirements that are demanded by the variety of regulatory authorities within EU or the USA. This activity is highly fostered and facilitated by a wide experience [LM1] in the dialogue and management of communications with such authorities in parallel to trial performance, which is of utmost importance given the wide scope of the trials conducted and the information generated in this phase of development.
Related Resources

The Electronic Application Form (eAF) is a unified EU form used for human and veterinary MAAs, variations, and renewals. It combines PDF for input and XML for data transfer, enabling data import/export, auto-copy, self-validation, and access to standardized term catalogues (EUTCT)—streamlining regulatory submissions and reducing manual input.

Highlights video

Highlights video

Highlights video

Highlights video

Highlights video

Highlights video

Asphalion contributions to the fight against COVID-19
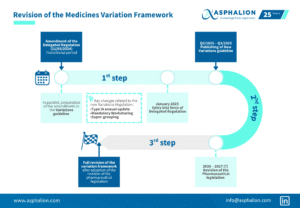
Embrace the Power of Smart Regulatory Strategy during this week! At Asphalion, we are dedicating this week to delving into the field of Life-Cycle Management.

Embrace the Power of Smart Regulatory Strategy during this week! At Asphalion, we are dedicating this week to delving into the field of Life-Cycle Management.

Embrace the Power of Smart Regulatory Strategy during this week! At Asphalion, we are dedicating this week to delving into the field of Life-Cycle Management, starting today with this infographic about the electronic application form (eAF).
Services

By department
By stage of development
By product
By region
Other services
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |