
ASPHALION´S HIGHLIGHTS | Second quarter 2024
Highlights video
The European Union represents an important marketing opportunity in terms of dimensions thanks to the existing common regulations among countries via the centralized procedures. The pharmaceutical regulation in this region is governed by supranational bodies, such as the EMA and its working parties and associate partner authorities.
To succeed in the approach to these entities, a correct designation of the product and a detailed strategy about the best way to contact, timings, meeting preparations, obtention of proper advice, management of CTAs, among others, are crucial to achieve an accelerated approval.
Europe Solutions
Schedule a free meeting!
Discuss your case with our experts and receive a valuable feedback
Share
Why Asphalion?
With 20+ years track record in handling submissions, Asphalion provides reliable support for all type of regulatory procedures in Europe. We provide regulatory strategy to anticipate any potential hurdle, coordinate your submissions and liaise with European agencies until the approval of the Marketing Authorisation.
Main benefits
Asphalion has large experience in writing, submitting and updating all types of technical dossiers specified for each product type including:
Furthermore, Asphalion has a proven track record and experience in following tracks, timings and standard formats in centralized submission procedures involving all intervenient regulatory entities.
Related Resources

Highlights video

Highlights video

Highlights video

Highlights video

Highlights video

Highlights video

IND preparation and submission business case: A small European biotech company focused on drug discovery and development interested in engaging with the FDA.

Asphalion contributions to the fight against COVID-19

A guide to have an overview of regional submissions requirements

(CTR) No 536/2014

A network of qualified partners that allows ASPHALION to provide pharmacovigilance services throughout Europe

Scientific & Regulatory Affairs Support during development of ATMPs & Biologics
Navigating Local Pharmacovigilance in European Countries, UK and Switzerland.
All what you need to know about the impact of MDR on Directive 2001/83/EC.
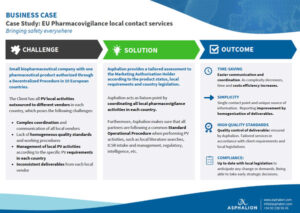
EU Pharmacovigilance local contact services case study. Small biopharmaceutical company with one
pharmaceutical product authorized through
a Decentralized Procedure in 10 European
countries.
The Client has all PV local activities
outsourced to different vendors in each
country, which poses several challenges.
At Asphalion, we are strongly committed to driving digitalization and integrating innovative tools to enhance our work. We believe that the thoughtful and responsible use of digital solutions, such as
Exciting News from Asphalion!

Asphalion expert Núria García participated last week in the 2nd Annual PragmaTIL Consortium Meeting in Amsterdam, hosted by the Netherlands Cancer Institute (NKI). This two-day event brought together partners from
Services

By department
By stage of development
By product
By region
Other services
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |