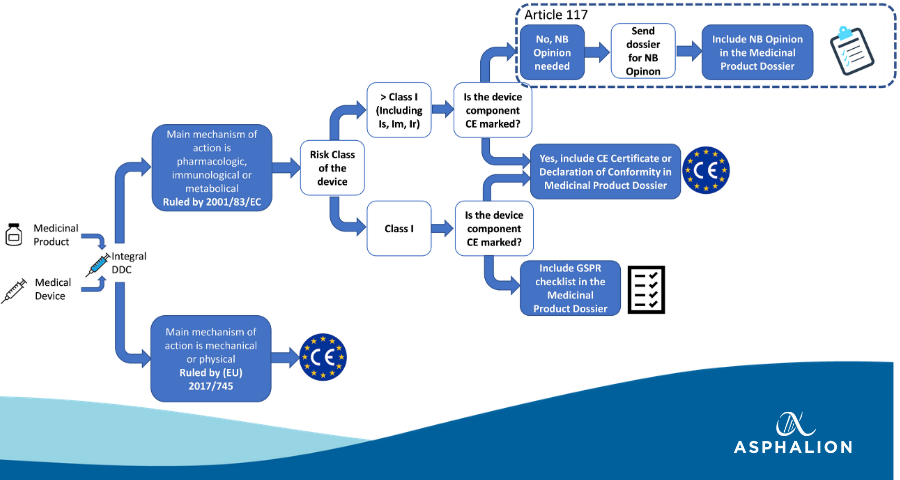
Starting on May 26th 2021, integrally-combined DDCs will fall under Article 117 of the MDR, which means that compliance of the device component with the applicable General Safety and Performance Requirements (GSPRs, Annex I of MDR) must be demonstrated, prior to submitting the initial Marketing Authorization Application (iMAA) for the DDC.
This new regulation will also have an impact on the life-cycle management of integral DDCs, including those already in the market, especially with regards to substantial changes of the device component.
if you have any questions do not hesitate to contact us at: [email protected].
👉 Medical Device Regulation (MDR) impact in Pharma Industry: Article 117
📅 28th April 2 p.m. CET (30’ webinar + 15’ Q&A)
👥 Asphalion Medical Device’s expert team
Registration: https://attendee.gotowebinar.com/register/1241665701524677644