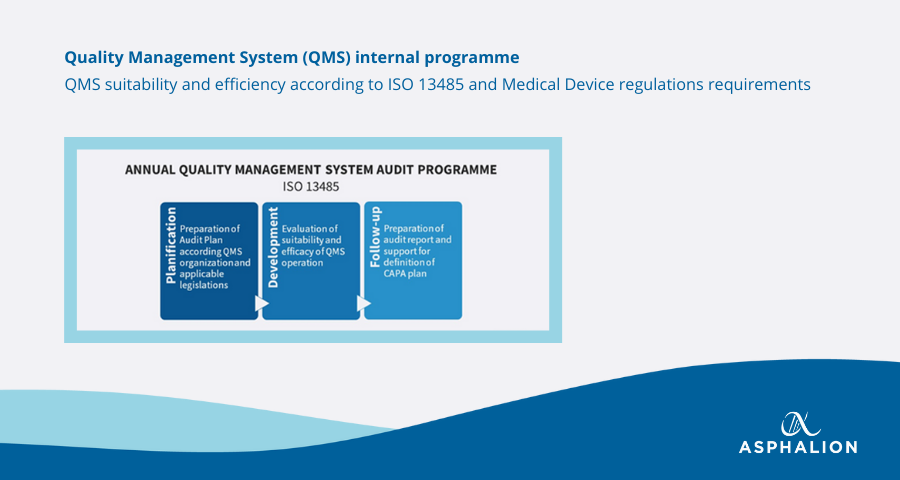
If you are currently planning the audit programme for this year and you are looking for support for the Quality Management System (QMS) internal audit, at Asphalion we count with Lead Auditors with a large track record and experience in the annual review of QMS suitability and efficiency according to ISO 13485 and Medical Device regulations requirements (EU MDR & IVDR and FDA 21 CRF Part 820).
We can support you in the three main stages of the audit process:
- Planification-> Preparation of Audit Plan according to QMS organization and applicable legislation
- Development-> Evaluation of suitability and efficacy of QMS operation
- Follow-up-> Preparation of Audit report and support for definition of CAPA plan
If you are interested in having further details, do not hesitate to contact us or book a free 30 min. meeting in the following link:
Additionally, you can send us an e-mail at: [email protected]