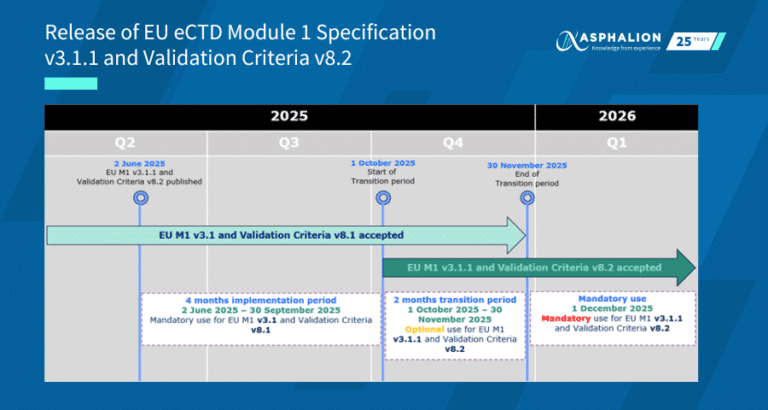
Have a look at this infographic that Extedo® and Asphalion have prepared with the summary updates in terms of publishing and eSubmission at a regional level. On this occasion, we are focusing on Switzerland!
Switzerland’s regulatory authority Swissmedic started with submissions for pharmaceuticals in eCTD format in 2009. Since 2010 Swissmedic is using eCTD M1 version 1.5. Additionally, Swissmedic is also accepting eDok format and paper submissions.
ASPHALION and our partner Extedo® can guide you with Swiss submissions. We can support you in a variety of activities.
For further information, you can contact us at: [email protected]