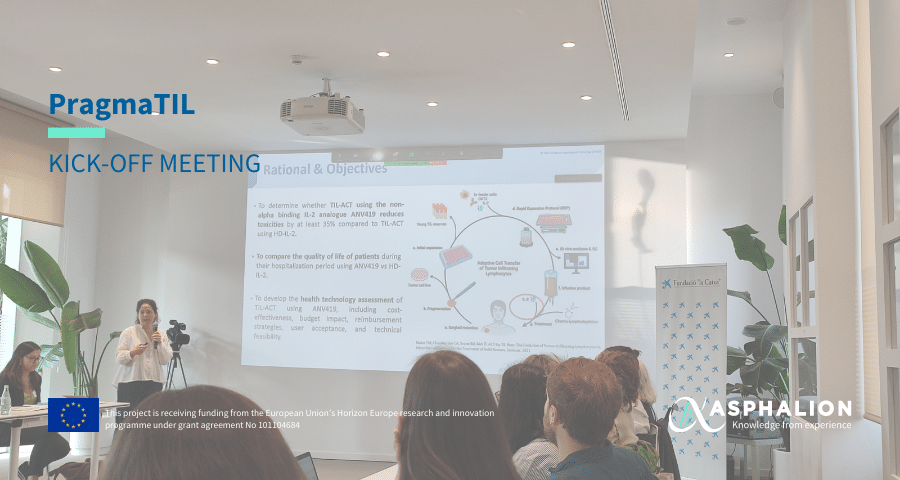
The launch of the EU-funded PragmaTIL project took place recently in Barcelona.
The PragmaTIL trial aims to optimize treatment of cancer patients with Tumour-Infiltrating Lymphocytes Adoptive Cell Therapy (TIL-ACT) and substantially expand and improve the clinical implementation of this treatment modality in academic hospitals. To this end, treatment related toxicities, associated to high-dose interleukin 2 (HD-IL-2) required for expansion and activation of TILs will be reduced while maintaining efficacy.
This improved tolerability will achieve a better clinical management of patients and enhance their quality of life, both of which represent major barriers for applying this treatment.
Asphalion will be in charge of the Regulatory part of the project, focusing on the submission of the clinical trial and the safety management plan.
Stay tuned for updates!
For further information, you can contact us at: [email protected]