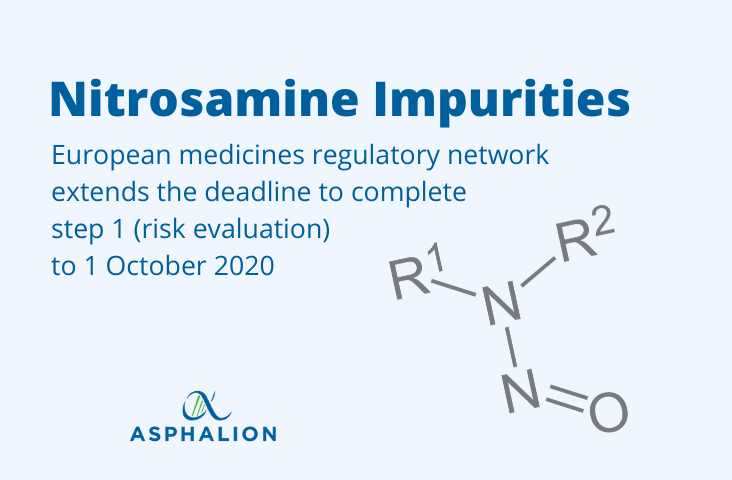
Due to the reports on the challenges encountered in meeting the original deadline of 26 March 2020, and the impact of the severe restrictions in place to combat the COVID-19 pandemic, the European medicines regulatory network has agreed to extend the deadline to complete step 1 (risk evaluation) to 1 October 2020.
The European medicines regulatory network encourages marketing authorisation holders (MAHs) to submit the outcome of step 1 before 1 October 2020 if they complete the risk evaluation or identify a risk in their products.
MAHs should:
- inform the national competent authorities as soon as possible if the tests confirm the presence of nitrosamine, regardless of the amount detected.
- evaluate the immediate risk to patients and take appropriate action to avoid or minimise their exposure to nitrosamines.
Asphalion can support in the preparation of the Risk Evaluation for Nitrosamine content. We offer a global service that covers risk analysis, analytical phase and includes regulatory support with variation management.
Contact us at info@asphalion.com.