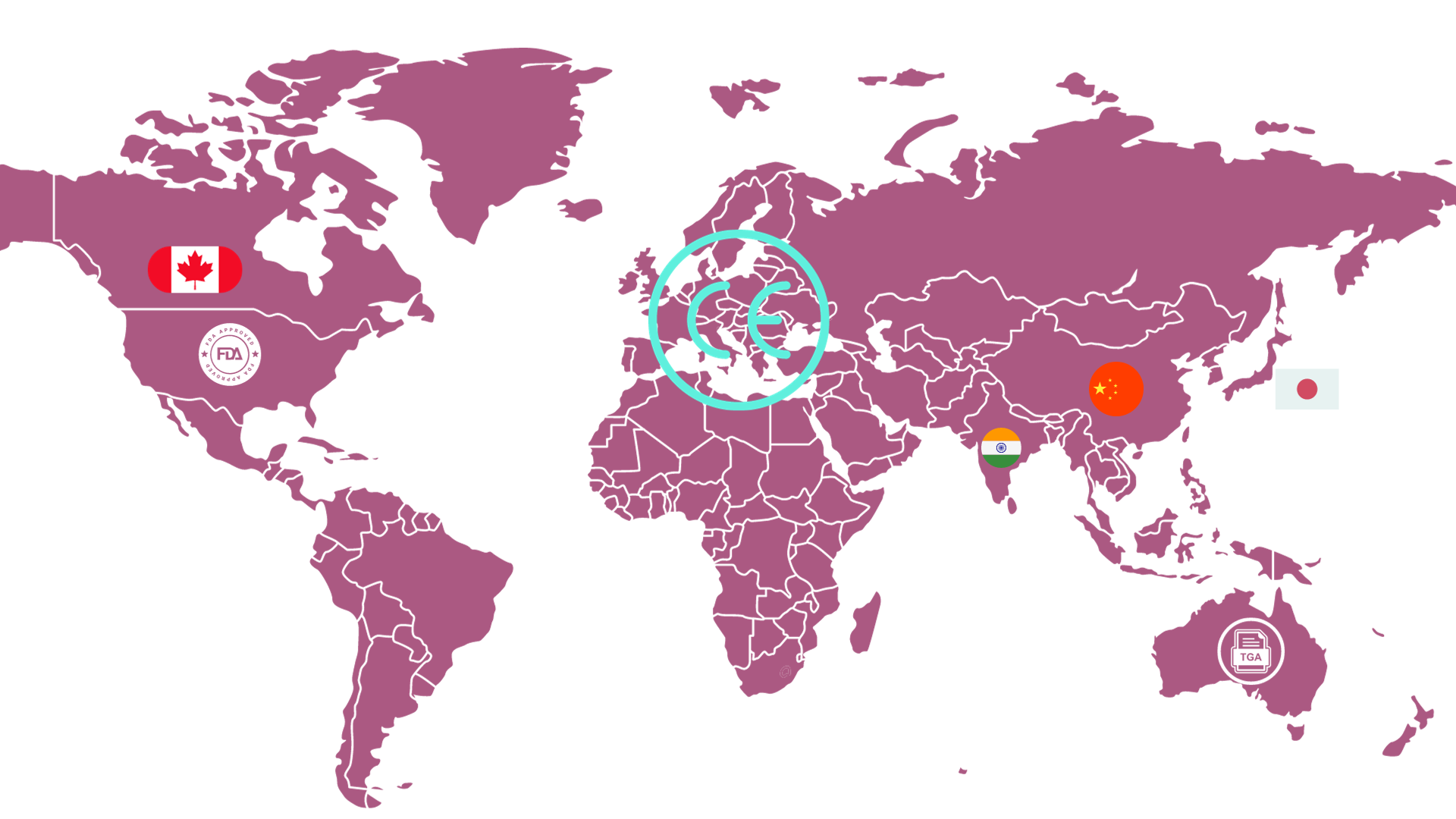
The medical device industry plays a pivotal role in advancing healthcare across the globe. With products developed and manufactured in one region often being marketed and sold internationally, it is crucial for medical device companies to understand the complex web of regulations that govern these products in different parts of the world
In this post, Adi reviews the key regulatory bodies for medical devices: United States, European Union, Canada, Japan, Australia, China and India.
Have a look at the new entry here: https://www.asphalion.com/overview-of-medical-device-regulations/
For further information, you can contact us at: [email protected]
Or schedule a free 30-minutes meeting here: SCHEDULE A FREE MEETING HERE