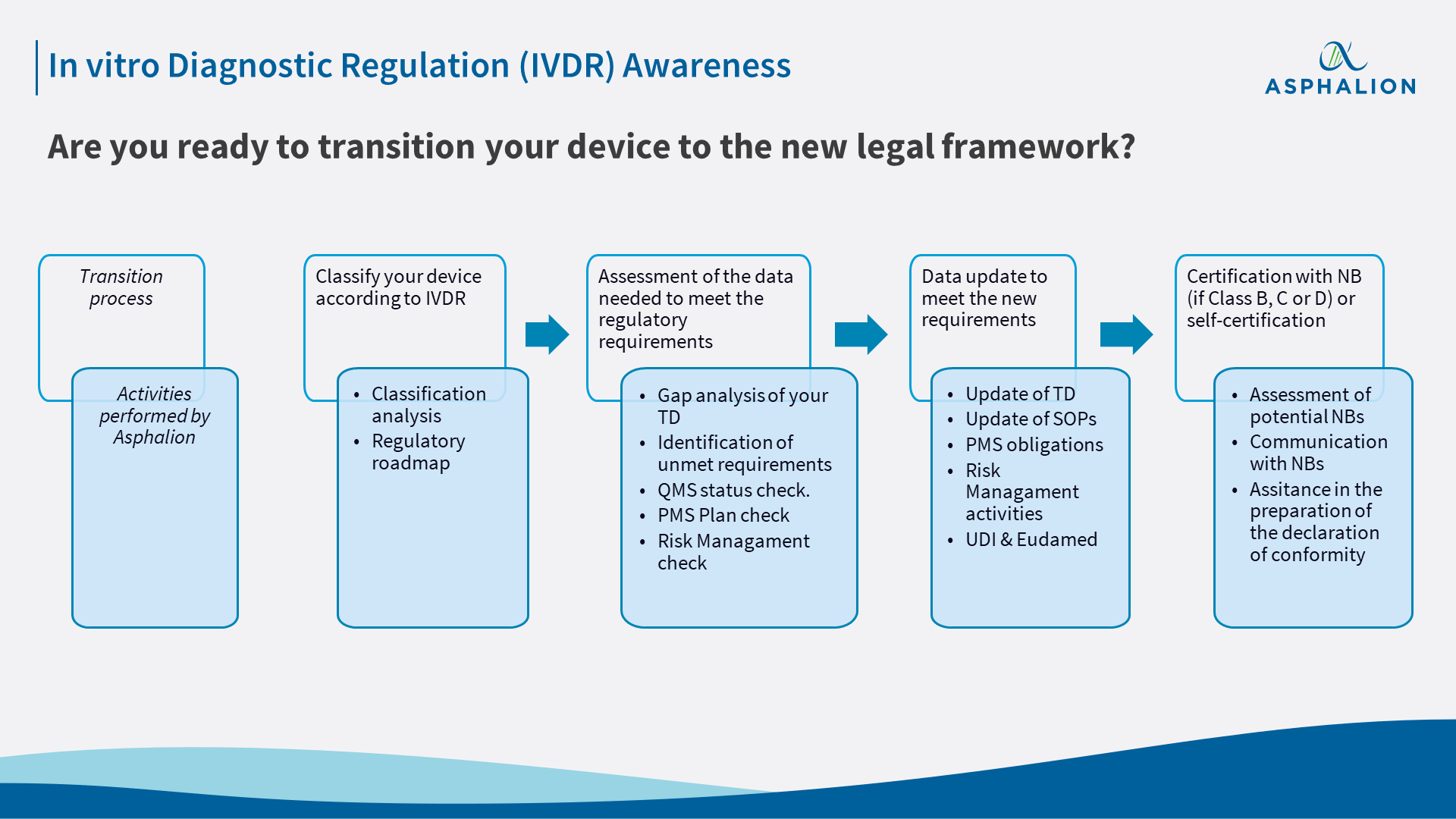
As of May 2022, IVDR will become fully applicable, displacing the old In vitro diagnostic directive (IVDD). Implementing IVDR will be much easier with a good action plan in place. Asphalion can aid you to ease this transition with diverse services tailored to meet your needs.
With the new classification rules introduced by the IVDR, it is quite likely that your device is classified as a Class B, C or D device. If such is the case, you will soon need to contact a Notified Body (NB) in order to obtain a CE certificate for your device, as you will no longer be allowed to place it on the market without the assessment of a NB. However, if your device has a valid certificate under the IVDD, you can still place it on the market until May 2024. Nevertheless, the QMS and post-market surveillance activities as laid down in IVDR will have to be met by 2022, and you should consider that after May 2024 it will be necessary to have certified your device under IVDR.