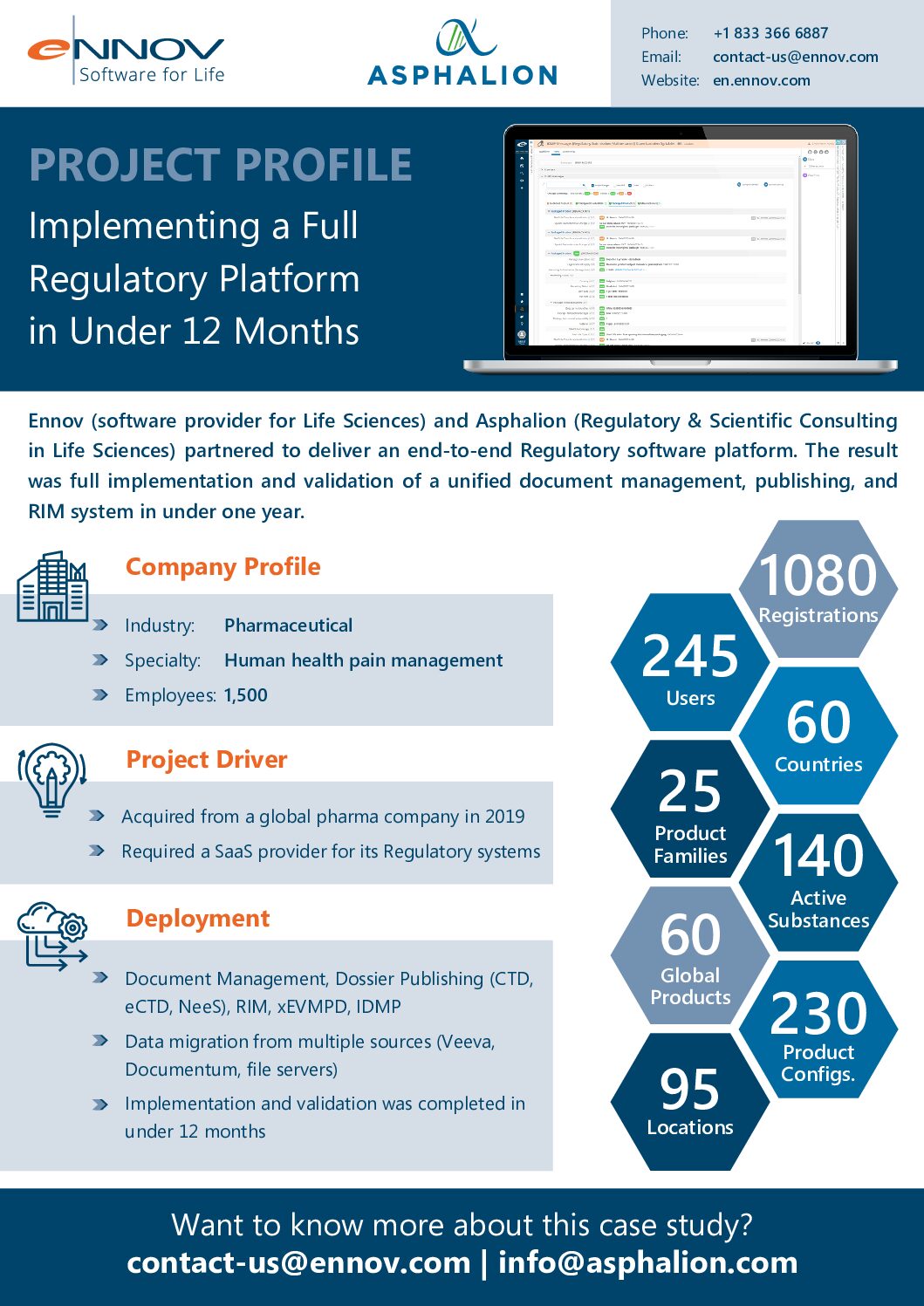
Did you miss us at the Global Pharmaceutical Regulatory Affairs Summit 2022 (GPRAS) last week?
No worries! See how Asphalion’s expertise paired with the Ennov platform can lead to incredible results.
Have a look at the downloadable project profile outlining the latest success of an Ennov and Asphalion implementation and conformity project.
Additionally, you can also contact us for further information!