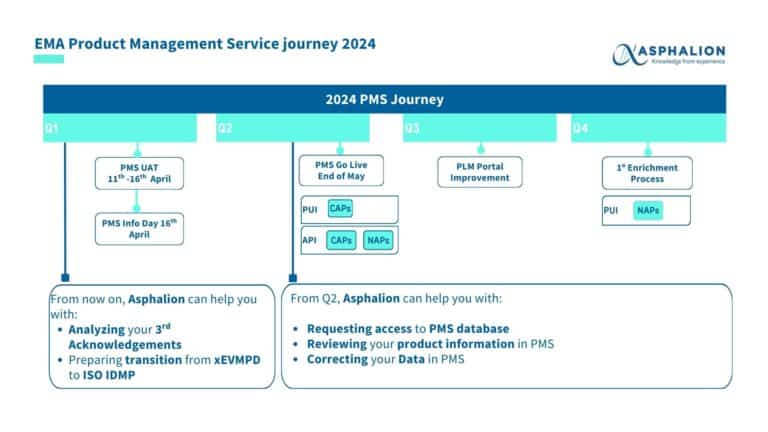
EVENT | EMA PMS Info Day
EMA is holding a new public event with the purpose to provide a comprehensive understanding of Product Management Service (PMS) and its implications on other EMA digital services with the objective to introduce the core concepts of PMS, ensuring that all attendees have the chance to understand its implementation.


















