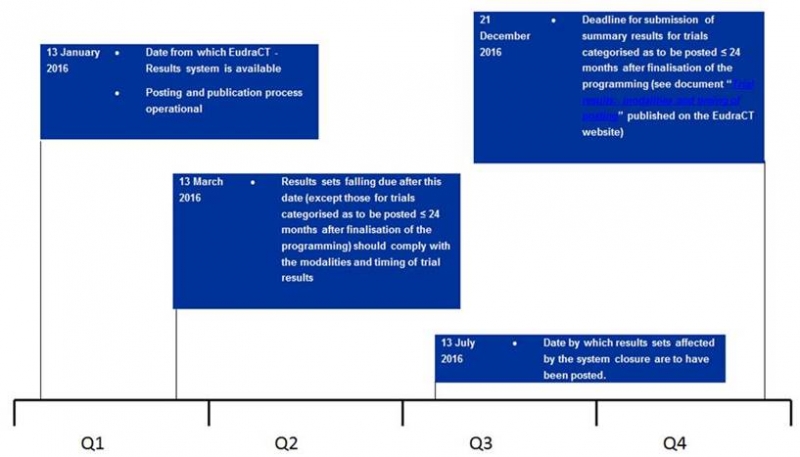
After 6
months of service withdrawal of EudraCT the new version 10.2.1.0 is now
fully operational.
This means
that the results of all your studies finalized between 31 July 2015 and 31
December 2015 have to be entered by 31 July 2016.
In
addition, for studies to be posted < 24 months after completion the deadline
is extended till the end of 2016.
With this
new version, EMA drives one step further to the achievement of Transparency in
Clinical Trials in Europe, whose summary results will be made publicly
available through the EU Clinical Trials Register (EU CTR).
Asphalion
can support you with the update of your studies into EudraCT.
Should you
request additional information, please do not hesitate to contact us at [email protected]