Good news regarding the fight against the Covid-19 pandemic! Today, EMA has recommended COVID-19 Vaccine Janssen for authorisation in the EU. This will be the fourth vaccine against SARS-CoV-2 to be authorized in Europe, which shall speed up the vaccination process along the continent.
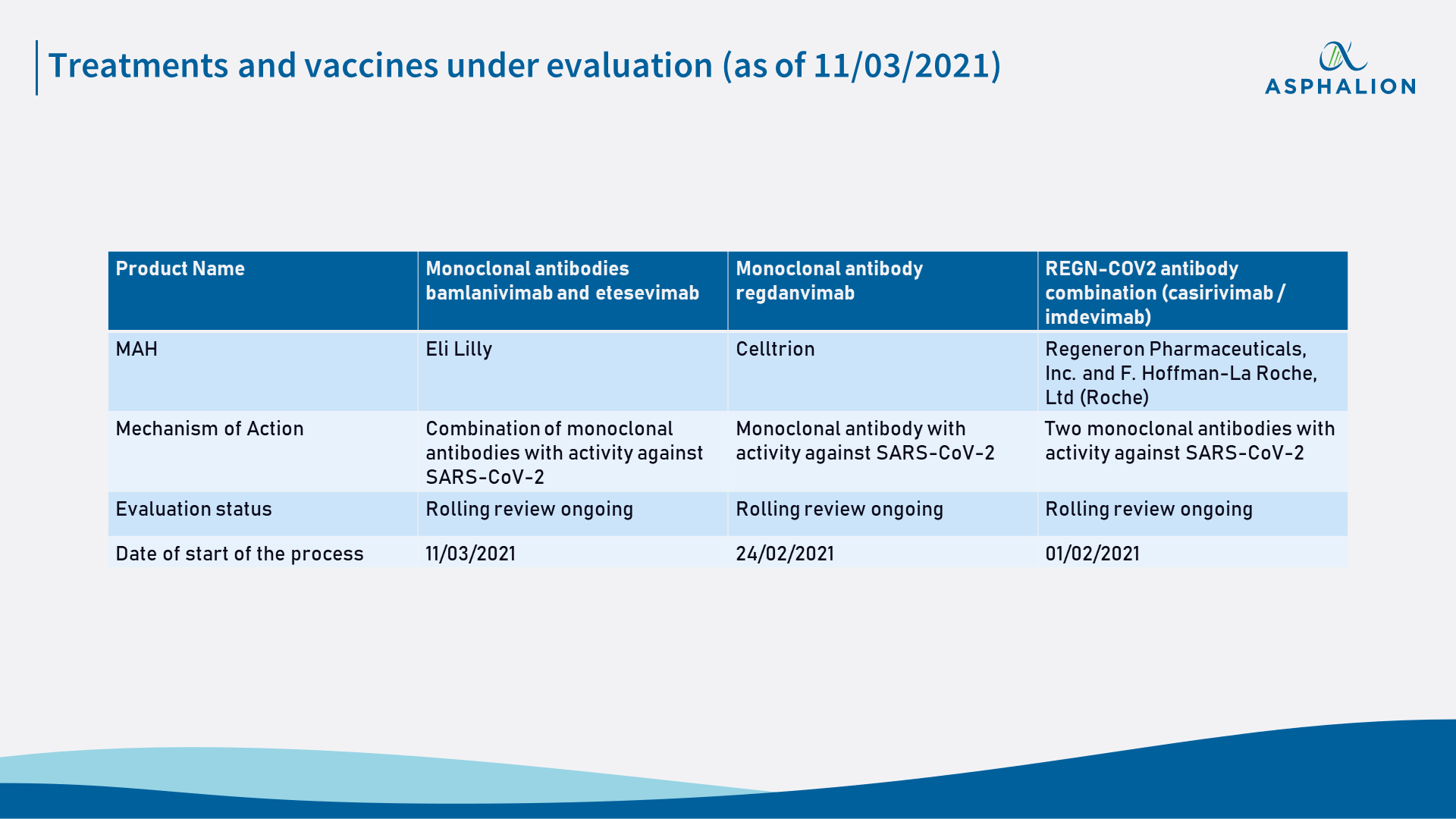
Additionally, EMA has also started today the rolling review of the monoclonal antibodies bamlanivimab and etesevimab (Eli Lilly). With this, there will be already three different treatments against COVID-19 under evaluation.
Stay tuned to be updated on all regulatory news related to COVID-19!
For further information you can contact us at: [email protected]