Have a look at a recent success story of how Asphalion helped an international European pharmaceutical company with a wide portfolio of medicinal products and important discrepancies between the registration documentation and the quality/manufacturing documentation, aiming to achieve full M3 dossier compliance.
Thanks to our extensive track record of over 20 years at Asphalion we can assist you to choose the right strategy, optmizing costs and enhancing time-to-market, and with all other kind of activities and projects.
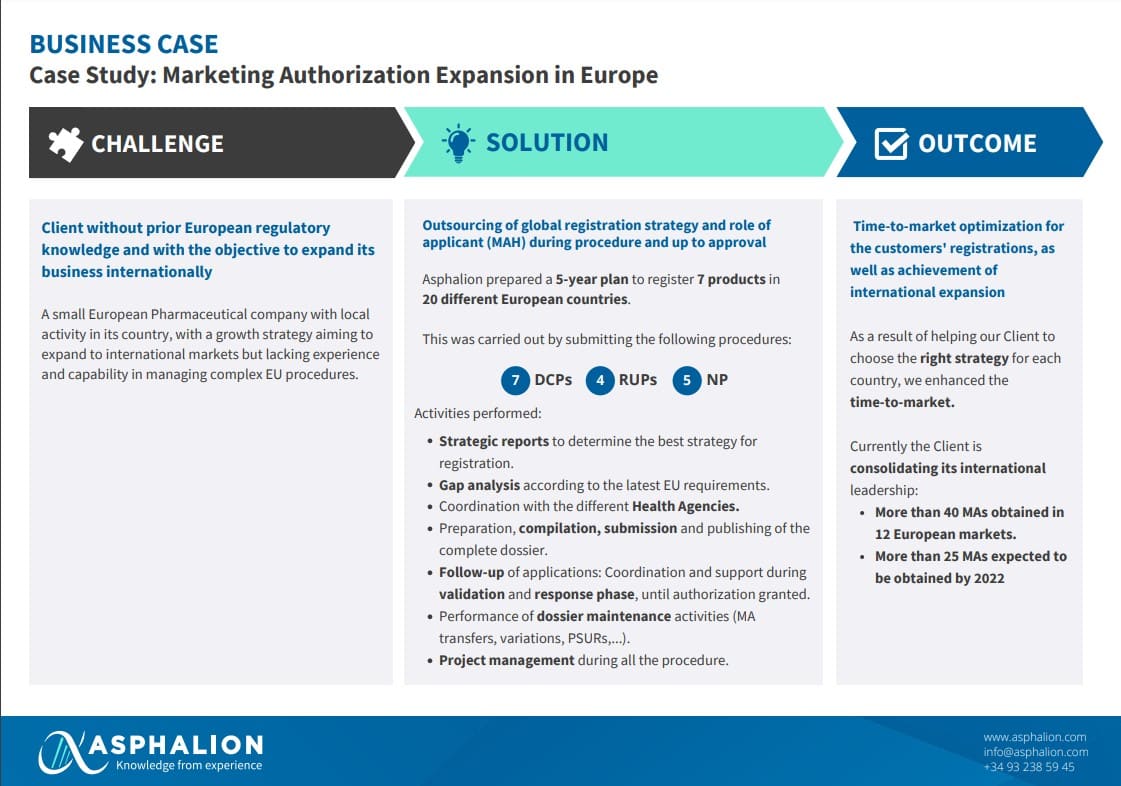
Case Study Asphalion_Marketing authorization expansion in Europe
For further information you can contact us at: [email protected]