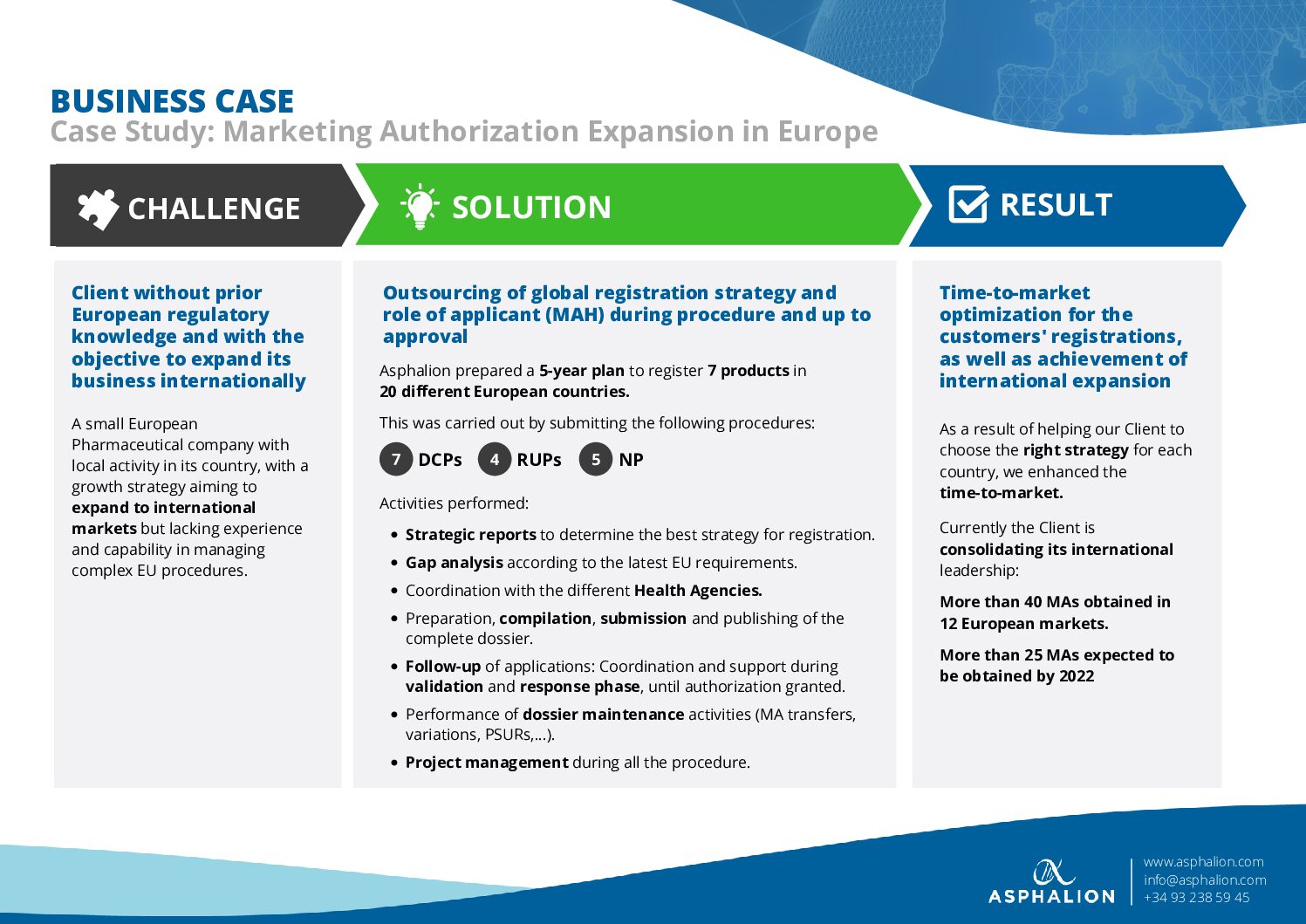
Registration rollouts within Europe can be complex, especially in terms of choosing the right strategy for each country.
Have a look at a recent success story of how Asphalion enabled a client to succeed with a multiple registration project in Europe. In this case, we jointly achieved over 40 MAs in 12 European markets, and 25 other approvals are expected to be finished this year.
At Asphalion we can assist you with your EU expansion projects, thanks to our extensive track record of over 20 years handling all types of procedures.
Click here to see the CASE STUDY.
For further information you can contact us at: [email protected]