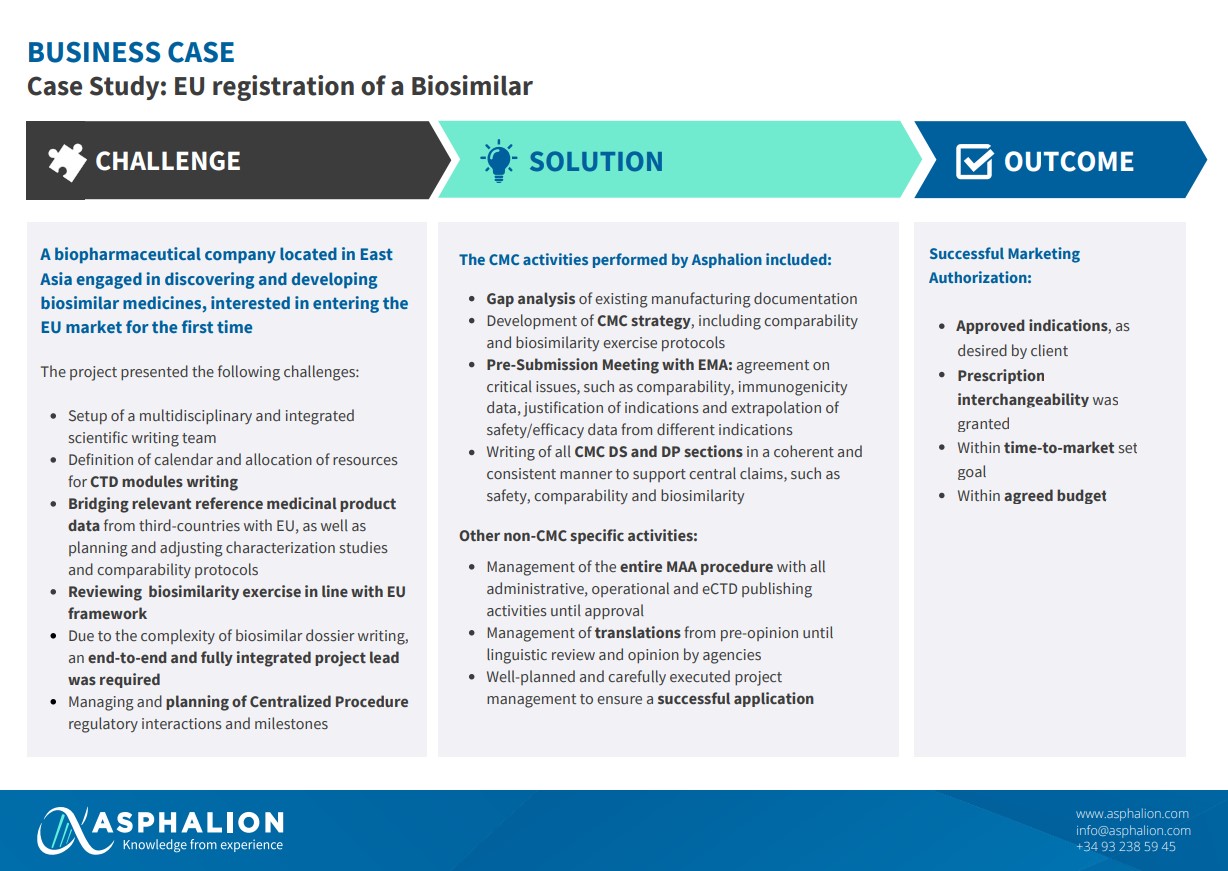
Have a look at a recent success story of how Asphalion achieved Marketing authorization for a biosimilar medicine for a company located in East Asia that wanted to access the EU market.
Thanks to our extensive track record of over 20 year at Asphalion we can assist you with your all CMC activities, non-CMC specific ones and with all other kind of projects.
CLICK HERE TO SEE THE CASE STUDY IN DETAIL
For further information you can contact us at: [email protected]