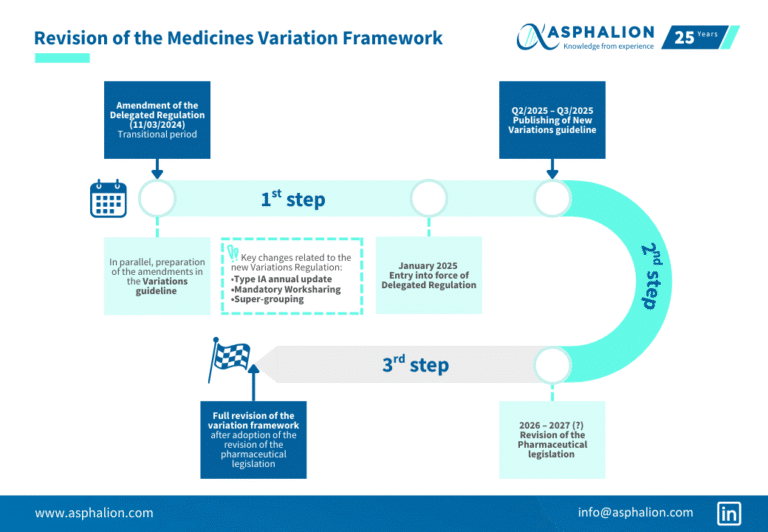
Did you know that…?
Our brand new web features a section called “SERVICES”. You can navigate through our services by choosing different approaches: by department, by stage of development, by product or by region. Today we are focusing on stage of development, more in detail on preclinical developments.
Prior to entering clinical trials to confirm the results obtained in preclinical research in humans, a product needs to be correctly defined to ensure the best regulatory strategy and clinical approach.
Facilitating a correct definition of the research development and regulatory plan, which involves proper designation of the product, will determine: timings, requirements during later phases, types of meetings to be carried out, the regulatory intermediaries that will be involved, and the possibility of accessing waivers and other mechanisms to accelerate approval that will depend on the medical need to be covered and the nature of the product.
Strategic regulatory inputs can be given from the very beginning for segmentation/niche indication, conditional approval/fast-track, orphan drug designations, and preclinical plans oriented for minimum time to market.
Asphalion can be the ideal regulatory partner during these early stages prior to proceeding to clinical research or in the very early stages of clinical research:
- Ad hoc Regulatory advice and strategic consultancy during prodcut development
- Support in early regulatory engagement with regulatory authorities including strategic and operative support during the meeting: preparation of Briefing Package, attendance to the meetings with authorities and adequation of the meeting content to comply with company plans
- Regulatory partner for Horizon programmes
Have a look here for additional, more detailed information:
https://www.asphalion.com/services/preclinical-development-stage/
For further requests, you can send us an email at: [email protected]