
ASPHALION´S HIGHLIGHTS | First quarter 2023
Highlights video
In spite of the existence of centralized procedures that span across all countries within the European Union, registration and commercialization of therapeutic products is sometimes focused on concrete countries based on companies’ strategic decisions. This usually involves an adaptation of marketing strategies to local regulatory frameworks and stakeholders for Decentralised Procedures (CDPs), Mutual Recognition Procedures (MRPs) and National-based procedures (NPs).
At Asphalion, we offer specific solutions based on each regulatory environment, both from the start of a new marketing authorization to the adaptation of a preexisting one to new markets. Asphalion has become a global Regulatory Affairs service provider with the capability to support your regulatory procedures in all EU countries at a local level through an expert, qualified network of partners with strong knowledge of local legislation and regulatory specificities. Asphalion´s team, together with native speakers will guide you to achieve the success in all your local duties as Marketing Authorisation Holder.
EU Local Solutions
Strategies to maximize price and reimbursement, when applicable.
Schedule a free meeting!
Discuss your case with our experts and receive a valuable feedback
Share
Why Asphalion?
Asphalion has become a global Regulatory Affairs service provider with the capability to support your regulatory procedures across the EU at a country level through an expert and qualified network of partners with strong knowledge of local legislation and regulatory specificities. Asphalion’s team, together with native speakers, will guide you to achieve success in all your local duties as Marketing Authorisation Holder.
Related Resources

Highlights video

A network of qualified partners that allows ASPHALION to provide pharmacovigilance services throughout Europe
Navigating Local Pharmacovigilance in European Countries, UK and Switzerland.
All what you need to know about the impact of MDR on Directive 2001/83/EC.
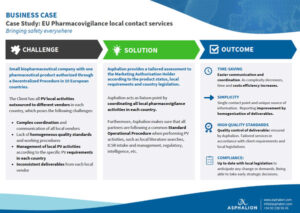
EU Pharmacovigilance local contact services case study. Small biopharmaceutical company with one
pharmaceutical product authorized through
a Decentralized Procedure in 10 European
countries.
The Client has all PV local activities
outsourced to different vendors in each
country, which poses several challenges.

This white paper examines the business drivers behind this shift and three factors that are critical to maximizing thebenefits of a unified RIM solution.

A lot of things have happened at Asphalion during these past three months, and we have summarized the highlights in a two minutes video. You can watch it here: Hope

At Asphalion, we are thrilled to announce the opening of our new office in Pamplona! This strategic decision reflects our commitment to being closer to our clients and strengthening our

At Asphalion we are very proud to announce that Nuria Garcia Pazos has been appointed EMA Subject Matter Expert (SME) for Clinical Trials Information System (CTIS)! She is the latest
Services

By department
By stage of development
By product
By region
Other services
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |