Resources Hub
Welcome to the Asphalion Resources Repository
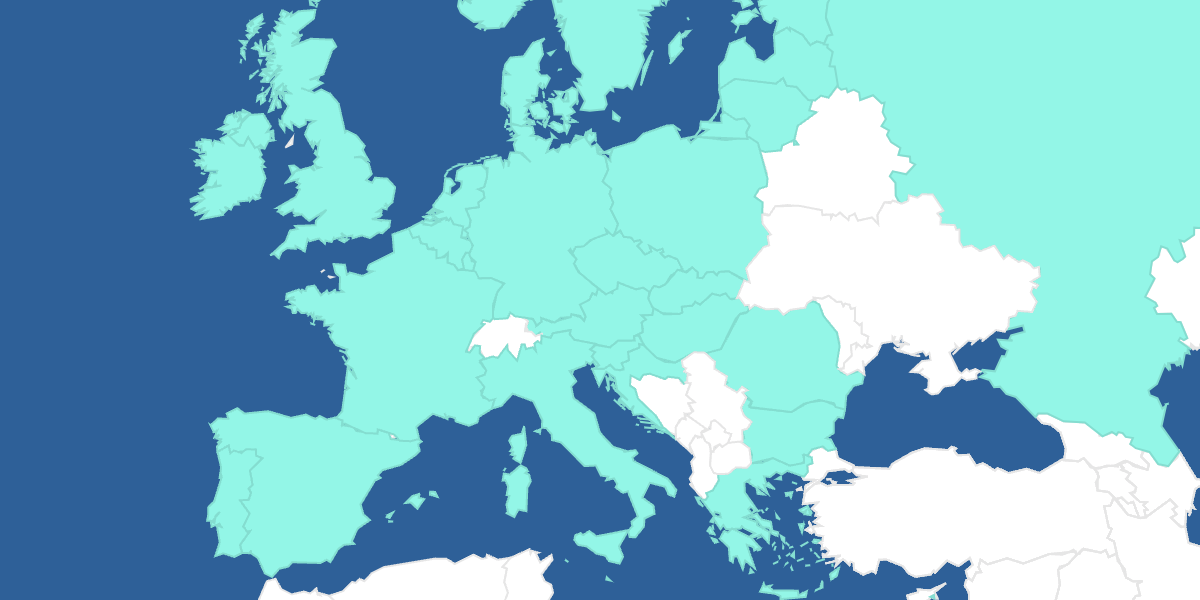
Asphanet Pharmacovigilance Interactive Map
Click on the countries to learn about the local safety requirements.
Glossary of Pharmaceutical
Regulatory Acronyms
Comprehensive glossary that provides clear definitions of pharmaceutical achronims.
Regulatory
Resources Library
Find here a collection of resources that can be useful in your day-to-day: Flyers, infographics, videos, webinars, whitepapers, etc.
We put at your disposal these useful and handy tools: