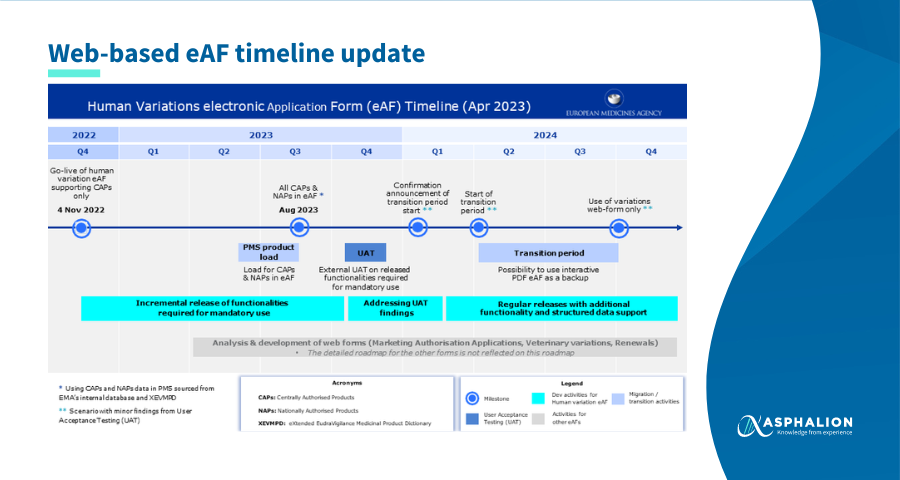
This update involves the following key milestones:
- June 2023 – CAPs and NAPs data transfer starting
- August 2023 – data transfer completion for all CAPs and NAPs
- November 2023 – User Acceptance Testing
- Q1 2024 – transition period starting date confirmation
- Q2 2024 – transition to mandatory use of web-based eAF for human variations
Please note that timelines to view migrated PMS product data in the Product User Interface on the PLM Portal will be announced in the future.
For further information, you can contact us at: [email protected]