
WEBINAR | Clinical Trials transition to CTR/CTIS
Ready to equip yourself with first-hand information from our experts? Join us in our new free webinar,

Ready to equip yourself with first-hand information from our experts? Join us in our new free webinar,
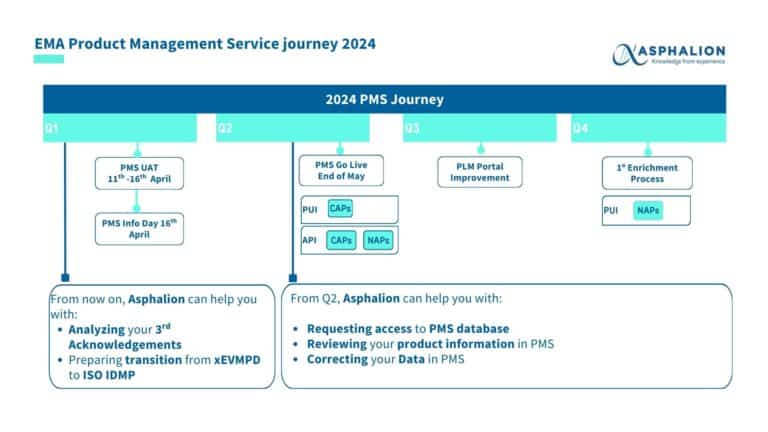
EMA is holding a new public event with the purpose to provide a comprehensive understanding of Product Management Service (PMS) and its implications on other EMA digital services with the objective to introduce the core concepts of PMS, ensuring that all attendees have the chance to understand its implementation.

During these two interactive days, actors of the healthcare community alongside world experts in biosimilar medicines will gather and debate the evolving biosimilar medicines landscape and emerging trends. Discussion will focus on sharing good practices and setting collective aims to achieve the depth, breadth and speed of the use of biosimilar medicines as a lever to untap their transformative value.

Article 17 of Regulation (EU) 2017/745 (Medical Device Regulation – MDR) regulates the reprocessing of single-use devices (SUDs) with relevance for the European Economic Area (EEA) which may only take place where permitted by national law and in accordance with this article.

At Asphalion, we are thrilled to announce the opening of our new office in Pamplona! This strategic

Want to dive deeper into our services, expertise, and regulatory affairs? Head over to our YouTube channel

Have a look at this Business Case where Asphalion provided a leading global pharmaceutical company with full

Seeking a regulatory ally to guarantee your company’s marketing materials comply with Spanish regulations? Achieve top-tier compliance
Search or filter
Services
© Asphalion
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |