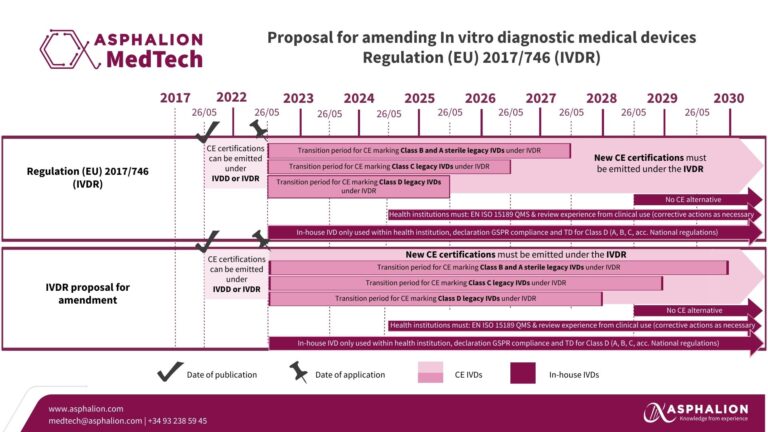
EC proposal on IVDs Regulation
Breaking Regulatory News: European Commission’s Proposal on In Vitro Diagnostic Medical Devices Regulation and EUDAMED Roll-Out

Breaking Regulatory News: European Commission’s Proposal on In Vitro Diagnostic Medical Devices Regulation and EUDAMED Roll-Out

The launch will bring several benefits, such as enabling quicker interaction between applicants and EMA, and improving data quality through integration with other EMA systems. Furthermore, the IRIS platform will allow applicants to monitor the status of their paediatric applications from any device and receive automatic updates on the status of their submissions. These features will significantly enhance the transparency and efficiency of the paediatric application process.

As a company, at Asphalion, we constantly endeavor to lead in embracing the latest trends. In this spirit, we wholeheartedly dedicate ourselves to integrating digital tools that streamline our processes, enhance our efficiency, and enable us to deliver unparalleled quality to all our clients.
May your day be filled with roses🌹 and books 📖 Happy Sant Jordi everyone from Asphalion! Feliç

At Asphalion, we’re dedicated to fostering a healthier world every day, and Earth Day 2024 is no
Asphalion fue galardonada con el Premio AEFI como empresa de menos de 250 trabajadores más comprometida con la formación de sus empleados por segundo año consecutivo

At our “People Talks” we feature some of the amazing people who make up our Asphalion family, so you can get to know them better and understand how their contributions shape our company’s success.

The partners of the PragmaTIL consortium have gathered in Barcelona to hold its first annual meeting. Asphalion’s
Search or filter
Services
© Asphalion
Schedule here a free 30-minutes meeting with one of our consultants and tell us about your project, challenges or doubts.
We will be happy to assist you!

| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-advertisement | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Advertisement" category. |
| cookielawinfo-checkbox-analytics | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Analytics" category. |
| cookielawinfo-checkbox-necessary | 1 year | Set by the GDPR Cookie Consent plugin, this cookie records the user consent for the cookies in the "Necessary" category. |
| cookielawinfo-checkbox-others | 1 year | Set by the GDPR Cookie Consent plugin, this cookie stores user consent for cookies in the category "Others". |
| CookieLawInfoConsent | 1 year | CookieYes sets this cookie to record the default button state of the corresponding category and the status of CCPA. It works only in coordination with the primary cookie. |
| elementor | never | The website's WordPress theme uses this cookie. It allows the website owner to implement or change the website's content in real-time. |
| viewed_cookie_policy | 1 year | The GDPR Cookie Consent plugin sets the cookie to store whether or not the user has consented to use cookies. It does not store any personal data. |
| wpEmojiSettingsSupports | session | WordPress sets this cookie when a user interacts with emojis on a WordPress site. It helps determine if the user's browser can display emojis properly. |
| Cookie | Duration | Description |
|---|---|---|
| _ga | 1 year 1 month 4 days | Google Analytics sets this cookie to calculate visitor, session and campaign data and track site usage for the site's analytics report. The cookie stores information anonymously and assigns a randomly generated number to recognise unique visitors. |
| _ga_* | 1 year 1 month 4 days | Google Analytics sets this cookie to store and count page views. |
| _gat_gtag_UA_* | 1 minute | Google Analytics sets this cookie to store a unique user ID. |
| _gid | 1 day | Google Analytics sets this cookie to store information on how visitors use a website while also creating an analytics report of the website's performance. Some of the collected data includes the number of visitors, their source, and the pages they visit anonymously. |
| Cookie | Duration | Description |
|---|---|---|
| VISITOR_INFO1_LIVE | 6 months | YouTube sets this cookie to measure bandwidth, determining whether the user gets the new or old player interface. |
| VISITOR_PRIVACY_METADATA | 6 months | YouTube sets this cookie to store the user's cookie consent state for the current domain. |
| YSC | session | Youtube sets this cookie to track the views of embedded videos on Youtube pages. |
| yt-remote-connected-devices | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt-remote-device-id | never | YouTube sets this cookie to store the user's video preferences using embedded YouTube videos. |
| yt.innertube::nextId | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |
| yt.innertube::requests | never | YouTube sets this cookie to register a unique ID to store data on what videos from YouTube the user has seen. |