On 18th June 2024, a new version 3.1 of the EU eCTD M1 specification was published on the eSubmission website, marking a significant milestone in the electronic submission of medicinal product dossiers not seen since July 2016 and February 2018 respectively.
What’s New in EU eCTD M1 Specification v3.1:
The version 3.1 sees the introduction of a number of changes across the specification, however, more specifically, the introduction of a new annex detailing the list of accepted file formats.
The other main changes correspond to clarifications on the use of related sequence, missing sections, duplicate documents already submitted, electronic signatures, addition of new submission types and units and clarifications, etc. To see the full set changes, please refer to the Release Notes and for the summary of the main changes refer to the table below.
| Section of the document | Change |
| File formats | Explanations added about the generally accepted file format (i.e. PDF) and the reference to the newly introduced Annex “EU Accepted file formats for eCTD”. Reminder: The generally accepted file format is the PDF, however, in specific cases, other formats will be exceptionally accepted in the eCTD modules |
| Envelope | If a particular sequence is being sent to a CMS for information only (for example, if the concerned strength is not registered in that country), no “envelope” element should be created for this CMS. However, it should be clear from the cover letter which CMSs are concerned. |
| New Submission Types and Units and clarifications | New submission type added (Article-18) New submission unit added (Re-examination) Clarification added for submission units “closing” and “consolidating” (including withdrawal clarifications) |
| Country & Language codes | New code “xi” (UK(NI)) and for “ga” (Irish) added |
What’s New in EU eCTD Validation Criteria v8.0:
The new validation criteria align with the new eCTD M1 Specification v3.1. The main changes correspond to the acceptance of new file formats, changes on file sizes, security settings, PDF settings and new picklist values. To see the full set changes, please refer to the Release Notes and for the summary of the main changes refer to the table below.
| Number | Details |
| 15.BP1.1 & 15.BP2.1 | The files provided in the folders for Module 1 to 5 are in acceptable formats |
| 15.BP1 & 15.BP6 | Individual files (except for video files) do not exceed 500 MB in size & Individual video files do not exceed 1GB in size |
| 15.11 & 15.12 | For all procedure types, the tracking table file is present in the correct location and correctly named |
| 16.3 | There are no further security settings applied to any individual file (except for files in Modules 1.0, 1.2, 3.3, 4.3, and 5.4) |
| 16.BP11 | New B/P validation rule added. For labelling documents (Module 1.3) the Description of the documents is filled in |
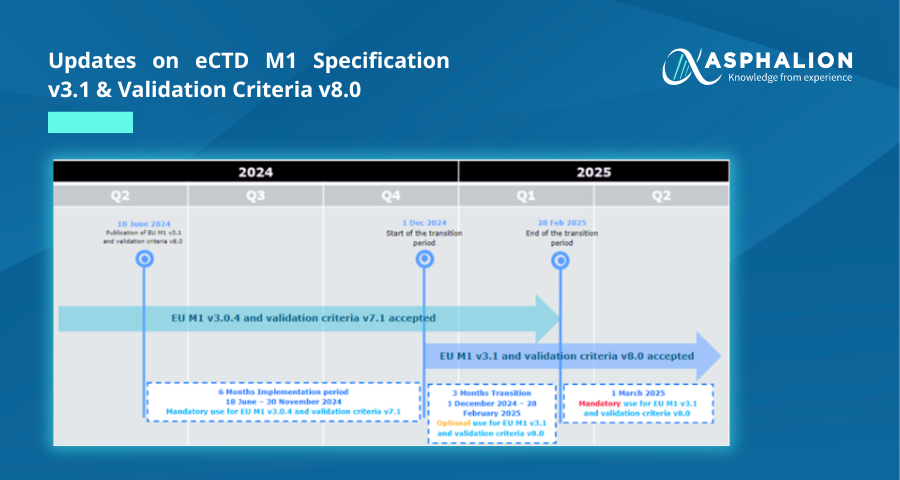
Transition Period:
- Initial Phase (18 June – 30 November 2024): Submissions must comply with EU M1 v3.0.4 and validation criteria v7.1.
- Intermediate Acceptance (1 December 2024 – 28 February 2025): Submissions may comply with either EU M1 v3.0.4 or v3.1 and validation criteria v7.1 or v8.0.
- Full Transition (From 1 March 2025): Only eCTDs compliant with EU M1 v3.1 and validation criteria v8.0 will be accepted.
Software Releases:
Applicants must wait until their corresponding eCTD publishing software providers releases these changes through their applications. We encourage to plan the next steps and be prepared for the software update and/or software validation (if applicable).
In case of EXTEDO’s clients, the changes will be implemented trough the upcoming versions 24.10, to be released by end of October, 2024.
Asphalion is proud to support our clients through these changes. We encourage all stakeholders to carefully plan their submissions to align with the new specifications and criteria. Our expert team is available to assist with any questions or support needed during this transition.
For more information, please contact us or visit the eSubmission website or email us at [email protected]