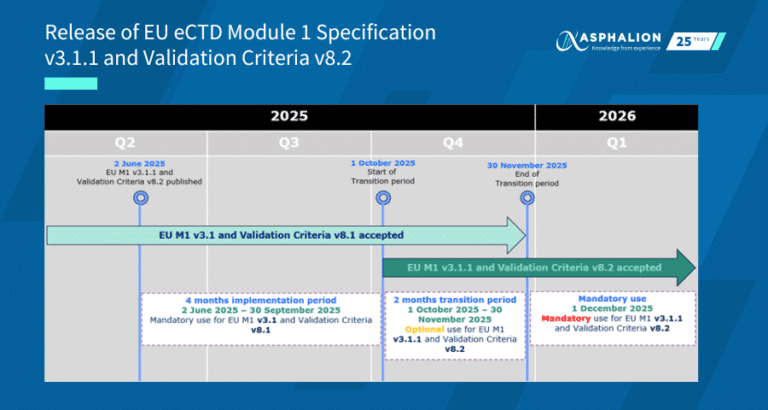
Last October 10th, the European Medicines Agency conducted the Webinar Product Management Service (xEVMPD) for Marketing Authorization Holders, in which important actions for MAHs were shared:
- Submit herbal and homeopathic products, as well as pending MRPs and DCPs to xEVMPD.
- Verify that non-CAPs have been properly migrated to PMS.
- Submit pack sizes for products under the ULCM (Unlicensed Medicinal Product List) before January 31, 2025, using consistent package descriptions for multilingual countries.
- Avoid referencing substances with SVG flag 0 and terms that need to be nullified in any new or existing product records in xEVMPD.
- Review received 3rd Acknowledgements and communications from EMA and take the required product amendment actions.
From Asphalion we could provide additional information and support in the following areas:
- xEVMPD Maintenance: Analyzing 3rd ACKs and submitting pack size structure data through xEVMPD.
- Data enrichment in compliance with ISO IDMP
- Reviewing your product in PMS
- Correcting your data in PMS
- Management of new tools on behalf of MAHs (PMS, RPM, eAF, ePI, etc.)
If you require any further information, contact us at: [email protected]