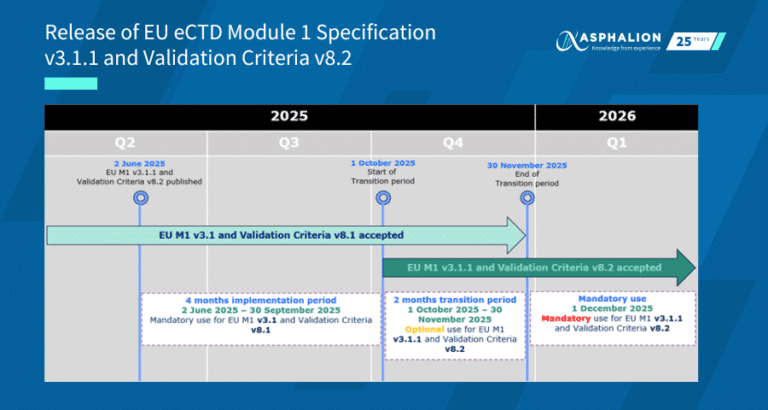
From July 11th, 2022 a new EMA Gateway portal is available, replacing the previous one that is no longer in use. The new portal (Syncplicity Web Client) erases the barrier of using Java as document explorer and is compatible with all internet browsers (not with Internet Explorer).
Please note that you will only be able to send submissions via the Syncplicity Web Client if you have successfully registered, received email confirmation with your login details and subsequently reset your password in Syncplicity. More information on the registration process and submissions can be found in EMA guideline (EMA/737304/2012), available at the link below.
The eSubmission Gateway Web Client is an electronic submission channel that allows the applicants to submit documents supporting all types of applications to the Agency securely over the internet in any electronic format (structured or non-structured). The eSubmission Gateway Web Client users will benefit from an automated confirmation of the technical validation feedback (eCTD only) and an automated upload to the Agency’s eCTD review system. The use of the eSubmission Gateway and the Web Client is mandatory for all human and veterinary submissions, including medical devices.
More information can be found in the European eSubmission webpage.
https://esubmission.ema.europa.eu/esubmission.html
For further information, you can also contact us at: [email protected]