The mutual recognition procedure (MRP) is the European Union-wide procedure through which a granted marketing authorisation of a medicine in one European Union Member State is recognised in other EU countries.
The legal provisions covering the mutual recognition procedure for human medicinal products are contained in Directive 2001/83/EC.
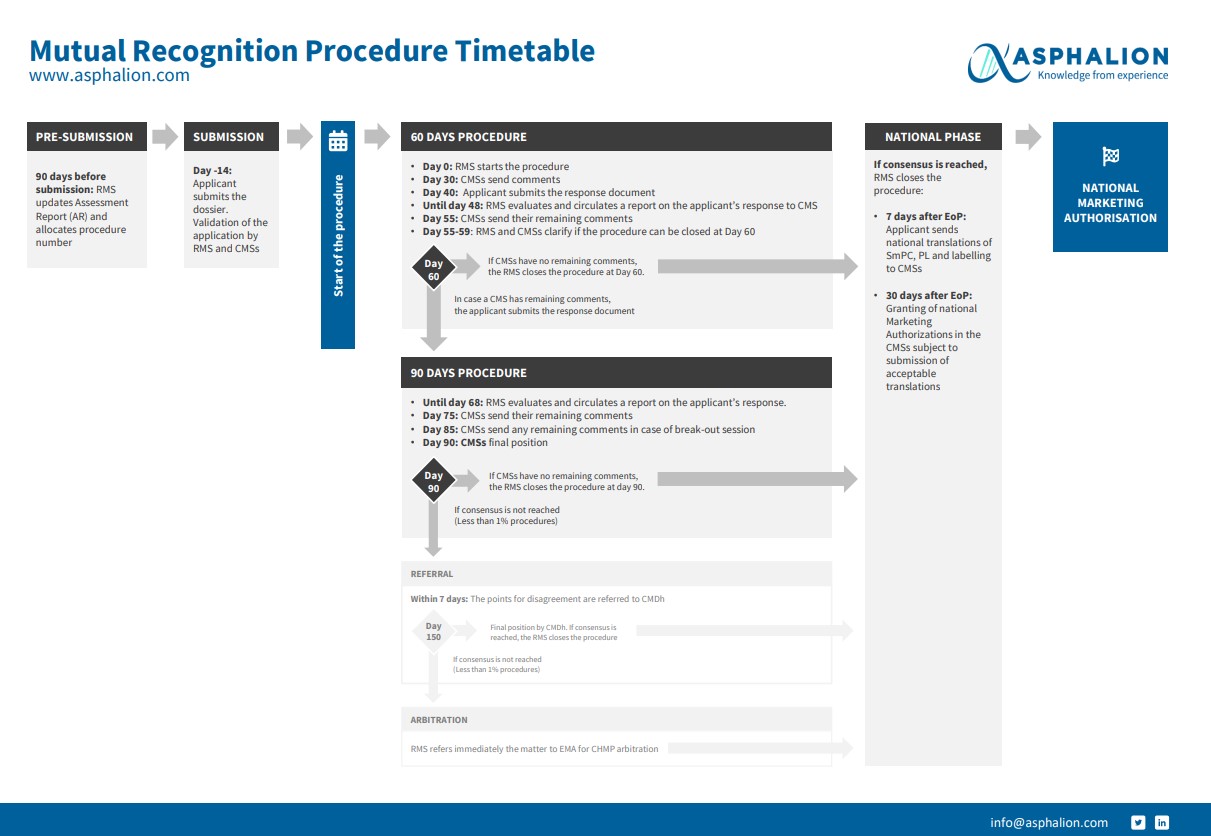
The process of a MRP is quite challenging and there are a lot of timelines to be kept.
We have summarized all MRP steps in this flow chart for you to download and keep always at hand. You can view it here:
For further information, you can contact us at: [email protected]