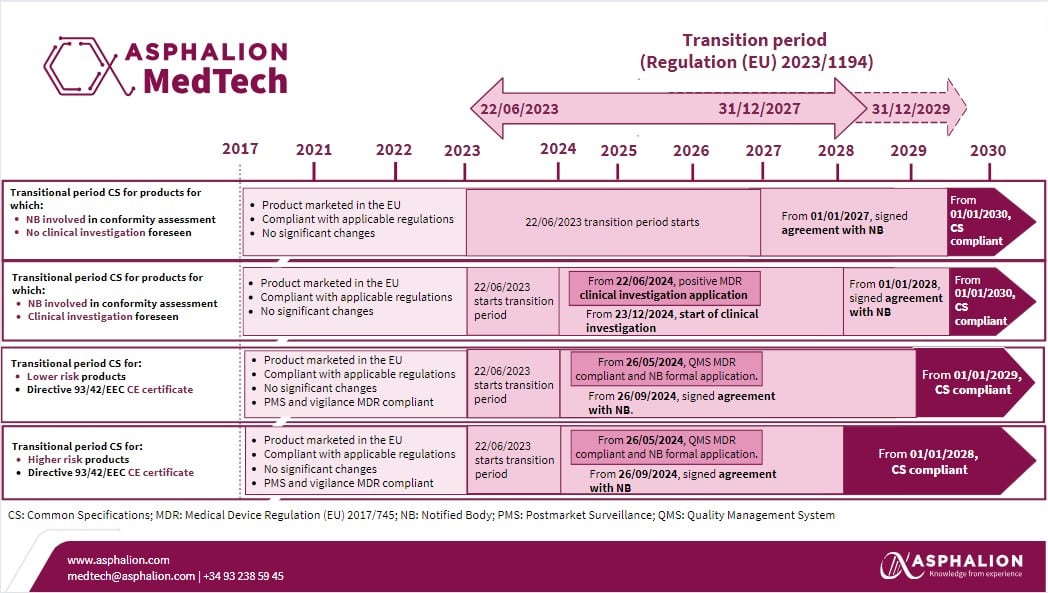
Regulation (EU) 2017/745 on medical devices (MDR) has been fully applicable since May 26, 2021, even encompassing products without an intended medical purpose as listed in its Annex XVI. Considering that transitional provisions set out in the MDR have been extended by Regulation (EU) 2023/607, the European Commission has recently released a Q&A document that provides clarity on transitional provisions for compliance with common specifications (CS) for Annex XVI products, as outlined in Commission Implementing Regulation (EU) 2022/2346 (CS), as amended by Commission Implementing Regulation (EU) 2023/1194.
Manufacturers of Annex XVI products are required to have implemented CS by June 22, 2023. However, specific transitional provisions have been defined to address particular situations, such as when a notified body needs to be involved in the conformity assessment procedure, when the manufacturer is considering conducting a clinical investigation followed by a conformity assessment procedure that involves a notified body, and when the Annex XVI product is covered by an MDD certificate that is no longer valid.
Have a look at the infographic our experts have prepared for you to have always at hand: MDR Annex XVI transition period
At Asphalion we are experts in MedTech.
Contact us for all your MedTech needs: [email protected]
You can also schedule a free 30-minutes meeting: https://bit.ly/3NwVsNR