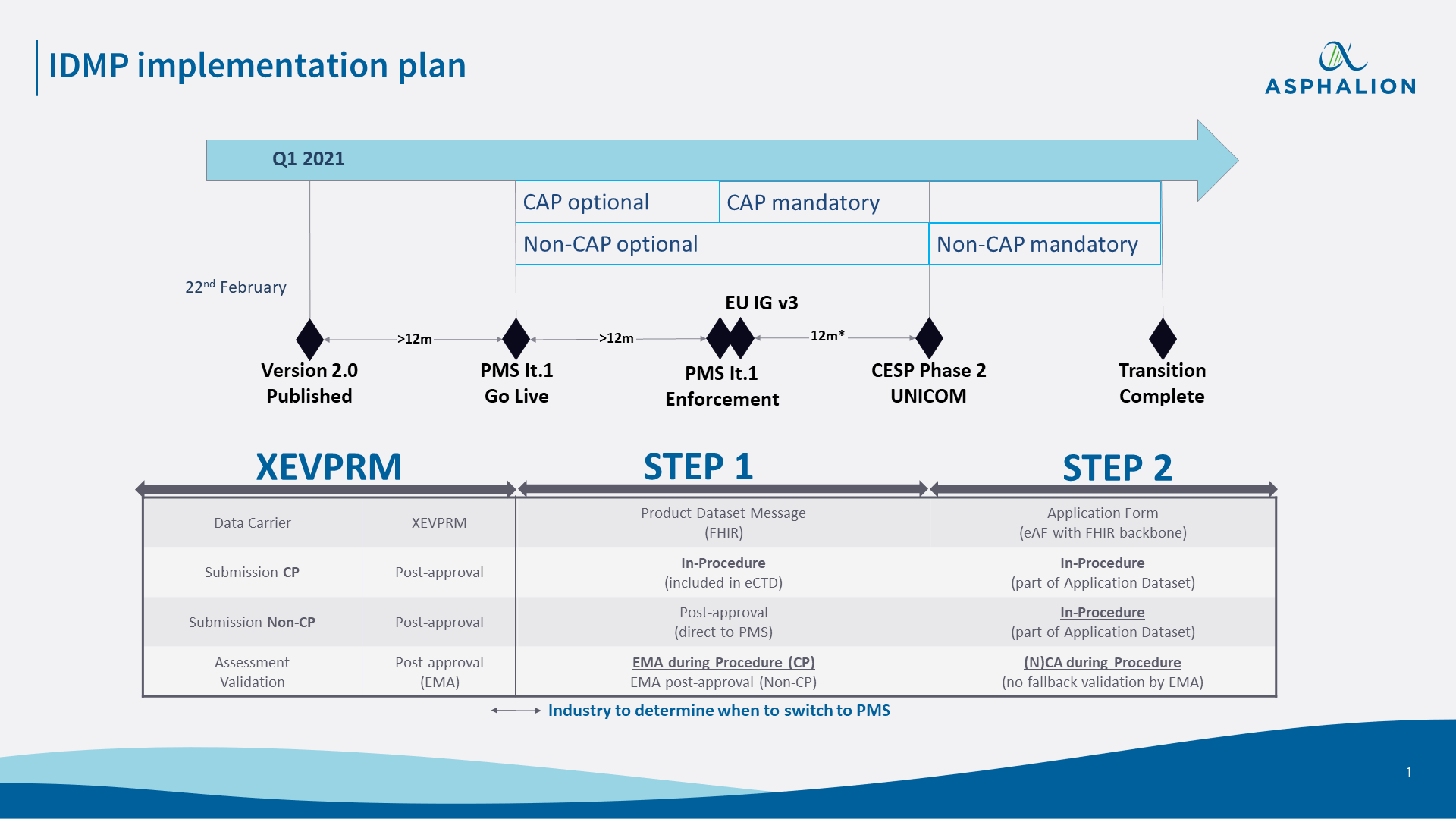
Version 2.0 of the European implementation guidance for IDMP has been finally published. Time is of the essence, so nothing catches you by surprise!
This version will not capture all industry comments, but it will cover the most important ones. Versions 2.1 and 2.2 are planned for Q2 and Q3 2021 respectively, with significant improvements and more examples.
This guide sets the clock start of a minimum of 12 months for the PMS (Product Management Service) go-live. A user interface (UI) will be released to manage data in the new format during the transition period, which will take at least another 12 months. After this period, submissions in IDMP format (FHIR) for centralised procedures (CAPs) will be mandatory. There will be an official publication at some point during this transition period which will mark the 12 months deadline until mandatory implementation.
In CAPs, the submission will be performed in FHIR format during the procedure, including it in the eCTD. The final message of the closing sequence is the one that will be uploaded to the PMS, and will feed xEVMPD.
For non-centralised procedures the process is not fully defined, but in principle in this first phase the submission in FHIR format will be performed post-authorisation and not within the eCTD.
Make sure to check the updated website and contact us if you need any further help or assistance.