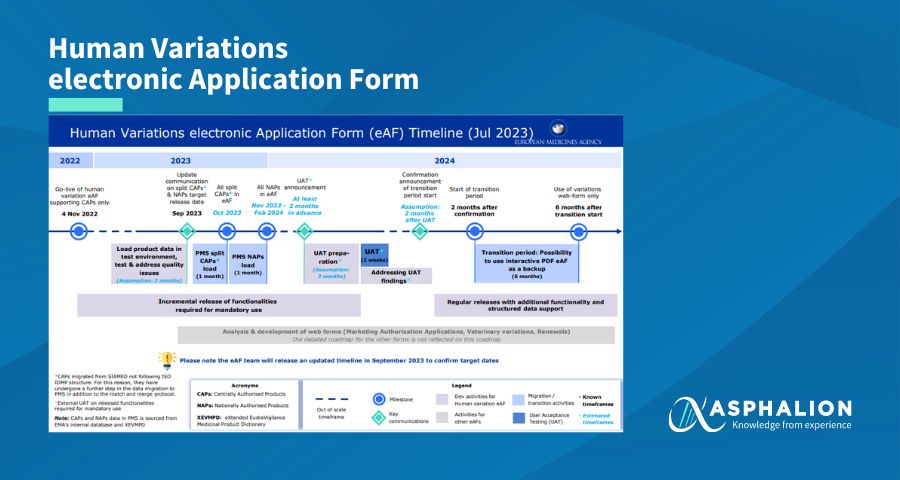
EMA continues working on the new web-based electronic Application Form (eAF). The priority is to ensure that the eAF is supported by a stable system with high-quality data and Product Management Service (PMS).
The new target dates are:
- October 2023: release of split CAPs
- November 2023 – February 2024: release of NAPs
Consequently, the rest of the key milestones on the PLM Portal will be rescheduled.
Please note that the eAF team will provide further updates to confirm the timeline in September 2023, based on the latest testing outcomes.
For further information, contact us at: [email protected]