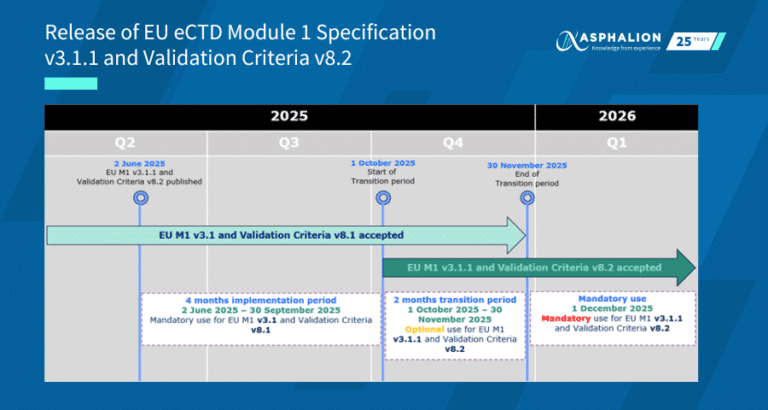
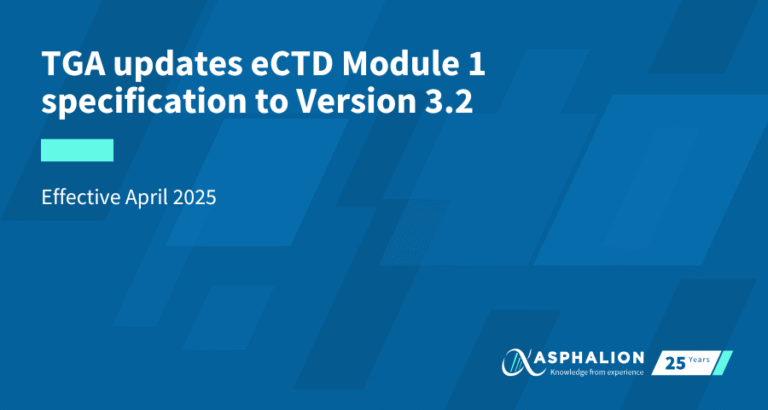
✦As of October 1st, 2020, the use of the Regulatory Enrolment Process (REP) is mandatory for pharmaceutical, biologic and radiopharmaceutical drugs for human use in Canada.
✦Starting today, Extedo® and Asphalion launch a monthly infographic to resume updates in terms of publishing and eSubmission at a regional level. This month we are starting with Canada!
✦ASPHALION and our partner Extedo® can offer you a wide range in Regulatory Services and Solutions in Canada. Asphalion can guide you with the transition to REP and with all the REP templates (RT, PI and CO) to be filed for each submission thereafter.
✦Stay tuned for the next updates!
✦Download the document with the flow chart here:
Health-Canada-Asphalion-Extedo
✦For further information, you can contact us at: [email protected]