The implementation and use of the electronic Aplication Form (eAF) and the RAEFAR electronic platform - 25th April at 10:00h GMT
FREE WEBINAR!!
eAF implementation and RAEFAR electronic platform
25th April at 10:00h GMT
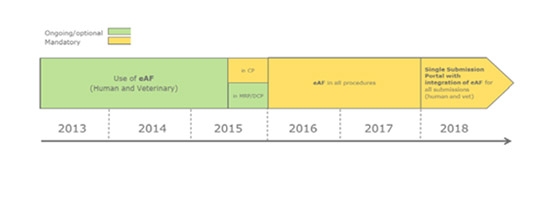
We would like to inform you that according to the Roadmap of the Heads of Medicines Agencies, the use of the Electronic Application Form (eAF) is mandatory from 1st January 2016 for all European
Procedures, including National Procedures.
The Spanish Agency (AEMPS) has adhered to it as well, meaning that the RAEFAR AF has been replaced by the eAF for new MAs, variations, renewals,.. Even though the use of the RAEFAR electronic platform is becoming voluntary, companies should take into account that RAEFAR is still the reference tool for consultation on the status of the procedures.
Additionally, RAEFAR is still mandatory for the following submissions:
- National code requests for additional formats for products authorized by MRP and DCP when they appear in the SPC
- Modifications of CP
- Request for free samples
- MA transfers
- “Fractionation” of texts during National Phases through the module “Gestión telemática de FichaTécnica y Prospectos” (this is the electronic platform of handling texts in Spain)
- Error corrections
- Requests for withdrawals and/or temporary suspensions of MA
Note: The management of these procedures did not change from the previous requirements.
Furthermore, the applicant is requested to confirm the administrative information through the RAEFAR electronic platform for administrative variations in order to have the procedure validated.
If you want to know more about eAF don’t miss our FREE WEBINAR, where
ASPHALION will be explaining the updates in the Spanish Legislation regarding the eAF and the RAEFAR electronic platform.
If you have any doubts regarding this or other topic, please contact ASPHALION at
[email protected]