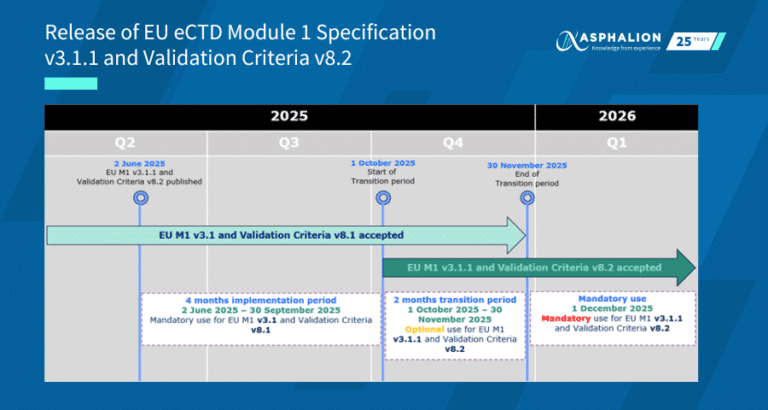
The GDUFA Self-Identification campaign is here!
As you may know, human generic drug facilities, sites, and organizations are required to submit identification information to the FDA once a year. This information should be submitted to the agency until June 1st in the Structured Product Labeling (SPL) electronic format.
Asphalion can provide you guidance and assistance along the process.
You can request further information by contacting us at: [email protected]