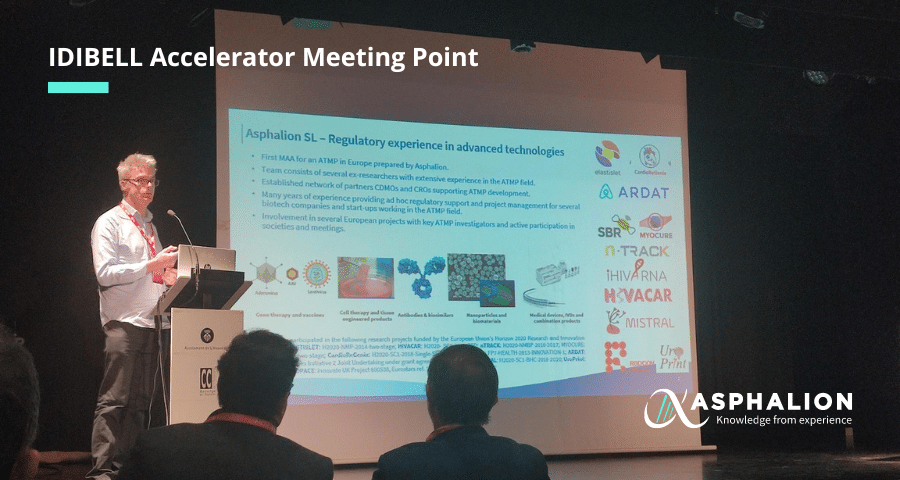
The 5th edition of IDIBELL’s Accelerator Meeting Point was held last week. The event is organized by the IDIBELL innovation and business development area and it has consolidated as a bridge between science and industry.
Christopher Mann, director of Development and Writing at Asphalion, gave a talk about “The importance of regulatory in ATMPs”. Chris stressed the importance of regulation in ATMPs and Medical Devicess from early stages of development.
IDIBELL is a member of the Campus of International Excellence of the University of Barcelona HUBc and is part of the CERCA institution of the Generalitat de Catalunya. In 2009 it became one of the first five Spanish research centers accredited as a health research institute by the Carlos III Health Institute. In addition, it is part of the “HR Excellence in Research” program of the European Union and is a member of EATRIS and REGIC. Since 2018, IDIBELL has been an Accredited Center of the AECC Scientific Foundation (FCAECC).
Need help in your regulatory endeavours? Contact us at: [email protected]