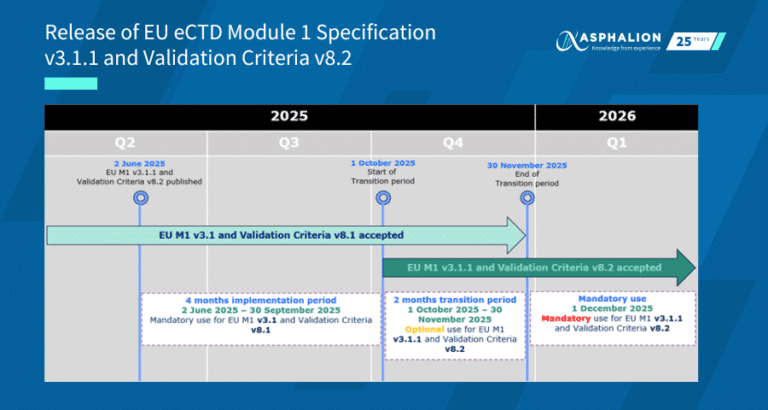
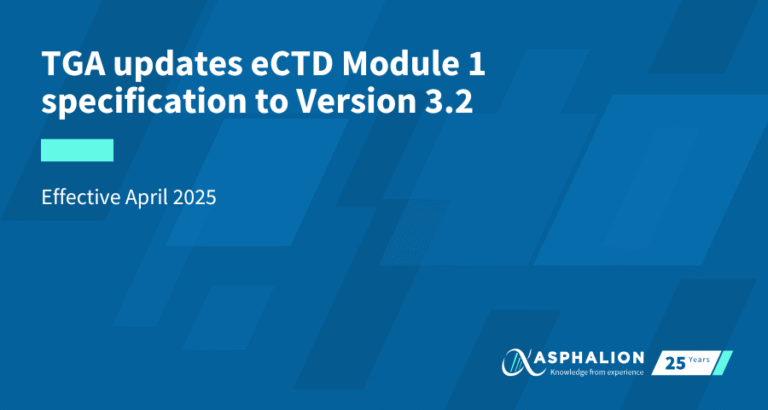
The European Medicines Agency (EMA) is upgrading security and usability by introducing email address authentication for its systems. This change simplifies access by eliminating the need for a username and password.
Key Dates to Keep in Mind:
- By January 20, 2025: All users are requested to opt-in to the new email authentication system.
- Conversion Timeline:
- January 20: Non-regulatory users without specific application roles.
- February 3: Non-regulatory users with application roles.
- March 3: Regulatory users within the European medicines regulatory network.
- March 31: All users will be converted.
How to Opt-In:
Visit the EMA Account Management page and follow the detailed instructions to opt-in. Once converted, you can use your email address for authentication, receiving either a redirect to an authentication page or an email with a code, depending on your user domain.
For further assistance or inquiries, you can contact us at: [email protected]