A revision of EU eCTD
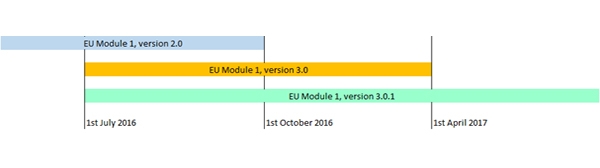
Module 1 Specification has taken place to satisfy several change requests submitted since version 2.0 of the Module 1 entered in to force, in September 2013. The published version 3.0 has been subsequently updated when errors were detected in the checksums and an updated version 3.0.1 is now available. Both versions v3.0 and v3.0.1 will enter into force on 1st July 2016. The versions 3.0 and v3.0.1 will replace v2.0 on 1 October 2016 after which eCTD sequences built based on EU M1 specification 2.0 version will no longer be accepted. For a parallel period of 6 months, from October 2016 until 1st April 2017 both versions will be accepted.
The use of version 3.0.1 will be mandatory from April 2017.
See below diagram for the implementation dates and validity period for the different versions of the specification.
Among the reasons behind the granting of a New Module 1 specification, there are:
- The Policy 0070 of Transparency required a new submission type.
- The use of CESP and EU Gateway with metadata and drive towards
automation of receipt with UUID (Universal Unique Identifier). - A new submission type was needed to support application on Certificates
of Suitability being submitted to the EDQM. - Name changes of Irish and Danish Agencies.
- The main features added as
new, or already existing modified, are the following: - Introduction of the Universal
Unique Identifier (UUID), a hexadecimal number of 32 digits which guarantees
that sequences are in the correct life cycle. This unique number will be
created automatically from by the Software, and should be generated when the
first sequence in eCTD format is created. The UUID will be kept as reference
for subsequence submissions. - Additional values for submission
types, among the possible choices already existing, in the envelope
information. These new fields will allow to have a better way to manage
regulatory activities. - For the Transparency Policy 0070 two new submissions type are
introduced: “redaction proposal version? and “final redacted document? - Introduction of the field “submission
units? which will describe actions within a specific regulatory activity. - The field of related sequencewill be always mandatory required.
- Addition of new submission typeto support application on CEP
- Introduction of EDQM as an additional
receiver to support the process of the CEP application
More details can be found in the eSubmission EMA web page:
http://esubmission.ema.europa.eu/eumodule1/index.htm
As a consequence, the validation criteria which relates mainly to the updated EU Module 1 has been updated, which only will affect tab sheet “eCTD Validation Criteria?. Among the main changes can be listed the following:
- Checking the field filled of related sequence which is always mandatory
- 9.7 EU regional XML, which checks the format of UUID if it is well
formed, according to ISO 11578:1996 - 9.8 EU regional XML, which checks the UUID is correct and the sequence
is being loaded into the correct eCTD Application - Updates to MD5s in comments etc.
Many other specific codes pertaining to the validation criteria have been updated. A comparison of the new major version 6.0 of validation criteria and the current version 5.0 can be download by following this link: