Timeline of the Proposal:
- On 23rd January 2024, the European Commission introduced an essential proposal concerning in vitro diagnostic medical devices (IVDs) and EUDAMED.
- The proposal was presented to the Council’s Working Party on Pharmaceuticals and Medical Devices on 30th January 2024.
- Following discussions, on 14th February 2024, the Committee of Permanent Representatives (Coreper) consented to the proposal’s text, paving the way for negotiations with the European Parliament.
- The file was promptly assigned to the ENVI committee in Parliament.
Expected Developments:
- Today, during the April II plenary session, the European Parliament has voted ‘YES’ on the Commission’s proposal under the urgent procedure (Rule 163 of the Rules of Procedure).
- An agreement has been reached that, should Parliament adopt its position at first reading matching the original proposal, the Council will endorse this position. This would mean the act is adopted in the wording that aligns with the European Parliament’s position.
Key Proposal Amendments:
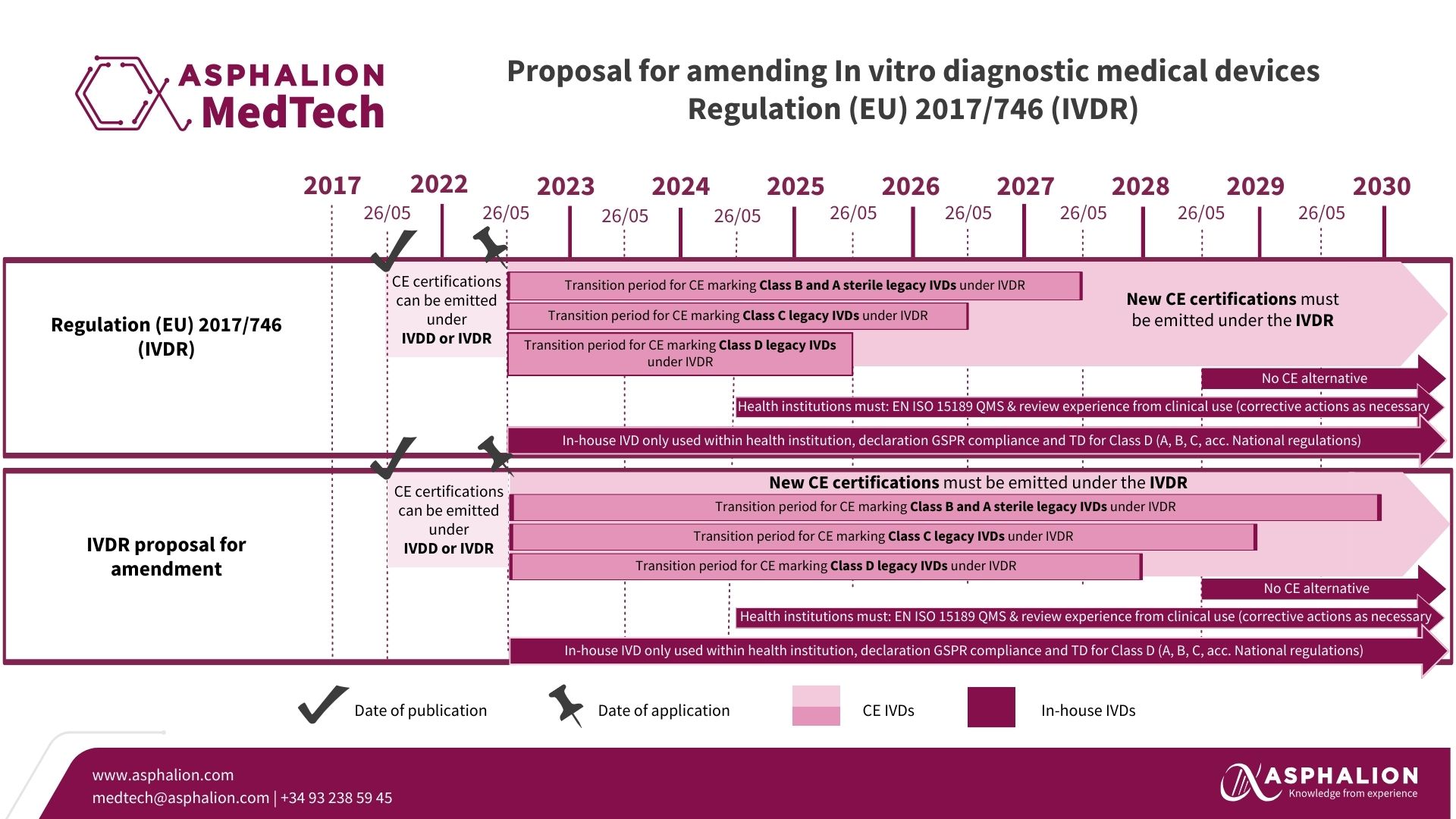
- In Vitro Diagnostic Medical Devices Regulation (IVDR):
- In light of the significant number of IVDs not yet compliant with Regulation (EU) 2017/746, the Commission proposes an extension of the transition periods. This is a critical move for the industry and provides manufacturers with the necessary time to meet the stringent requirements.
- EUDAMED Roll-Out:
- The proposal suggests a phased implementation of the electronic systems within the European database on medical devices (‘Eudamed’). This strategic approach allows for the use of completed modules rather than waiting for the finalisation of all six modules.
- Mandatory use of several modules could then start as early as Q4/2025.
Impact on the Industry:
The adoption of this proposal is poised to have a profound impact on the medical devices sector. Not only will it provide manufacturers with much-needed breathing space, but it will also ensure a smoother transition to the new regulatory environment.
Official Publication:
Once the regulation is adopted, it will come into effect on the same day it is published in the Official Journal of the European Union.
Read the complete new here: Commission welcomes adoption by European Parliament of measures to improve the availability of in vitro diagnostics
Stay tuned as we continue to monitor this development closely and provide you with further insights.
For any assistance or questions regarding these changes, please feel free to reach out to us at: medtech@asphalion.com