As we highlighted in our recent webinar “Review of Hot Topics in Regulatory & Safety + Outlook into 2024,” significant changes are on the horizon for CMC in 2024! These updates will shape the future of analytical procedure development and validation, as well as advanced manufacturing technologies.
Key Changes coming this year:
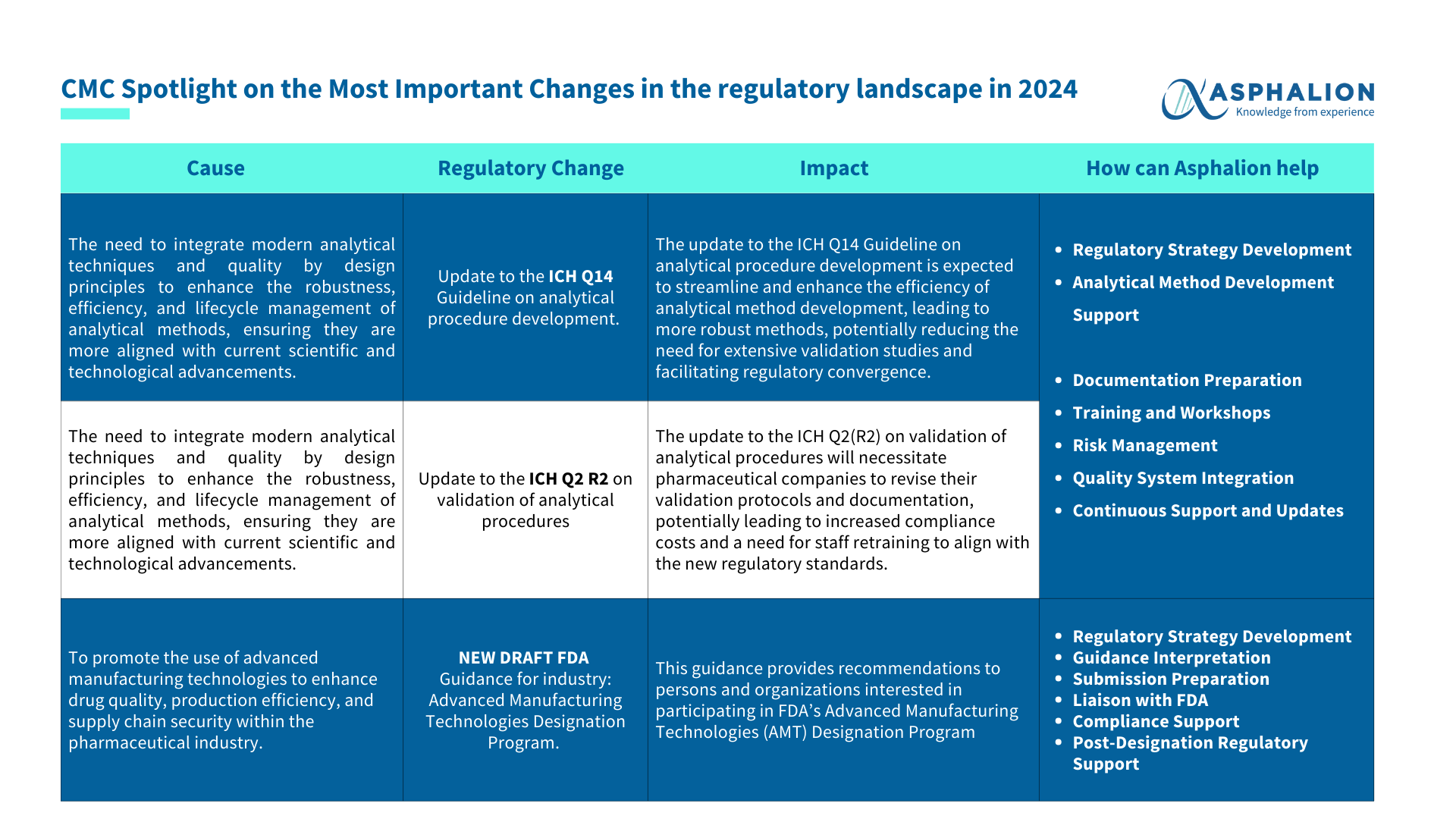
- Updates to Critical ICH Guidelines:
In 2024, expect important updates to ICH guidelines that will impact how analytical procedures are developed and validated. These changes are crucial for maintaining high standards and ensuring compliance within the industry.
- New FDA Draft Guidance for Advanced Manufacturing Technologies:
The FDA is introducing a new draft guidance for the Advanced Manufacturing Technologies Designation Program. This guidance offers fresh opportunities for innovation in the industry, providing a pathway for adopting advanced manufacturing technologies.
Our team of experts has meticulously reviewed these changes to provide you with the latest insights and actionable guidance. We offer tailored solutions to help you navigate these updates seamlessly, ensuring compliance, optimizing your processes, and adapting to the evolving regulatory landscape.
How We Can Help:
- Regulatory Strategy Development: Crafting robust strategies to align with regulatory changes.
- Analytical Method Development Support: Assistance in developing and validating analytical methods.
- Documentation Preparation: Comprehensive support in preparing all necessary regulatory documents.
- Training and Workshops: Providing educational resources to keep your team updated and compliant.
- Risk Management: Identifying and mitigating potential risks associated with regulatory changes.
- Quality System Integration: Seamless integration of quality systems to meet new guidelines.
- Continuous Support and Updates: Ongoing support to ensure continuous compliance with evolving regulations.
- Guidance Interpretation: Clarifying and interpreting new guidelines for practical application.
- Submission Preparation: Assisting in the preparation and submission of regulatory documents.
- Liaison with FDA: Acting as a bridge between your organization and US regulatory authorities.
- Compliance Support: Ensuring your processes and products meet the latest regulatory standards.
- Post-Designation Regulatory Support: Providing ongoing support after regulatory designations are achieved.
If you need support or have any questions, feel free to contact us at: [email protected]
Follow us for more in-depth information and updates on the key changes ahead. Together, we can navigate the future of CMC with confidence and precision.