The centralised procedure (CP) is the European Union-wide procedure for the authorisation of medicines, where there is a single marketing authorisation application to EMA, a single evaluation and a single authorisation. Only certain medicines are eligible for the centralised procedure.
This procedure allows the marketing authorisation holder to market the medicine and make it available to patients and healthcare professionals throughout the EU on the basis of a single marketing authorisation.
The centralised procedure is compulsory for:
- Human medicines containing a new active substance to treat:
- Medicines derived from biotechnology processes, such as genetic engineering
- Advanced-therapy medicines, such as gene-therapy, somatic cell-therapy or tissue-engineered medicines
- Orphan medicines (medicines for rare diseases)
- Veterinary medicines for use as growth or yield enhancers
Today, the great majority of new, innovative medicines (including COVID-19 vaccines) pass through the centralised authorisation procedure in order to be marketed in the EU.
However, under EU law, EMA has no authority to actually permit marketing in the different EU countries. The
Once granted by the European Commission, the centralised marketing authorisation is valid in all EU Member States as well as in the European Economic Area (EEA) countries Iceland, Liechtenstein and Norway.
Commission decisions are published in the Community Register of medicinal products for human use.
The benefits of the centralised procedure for EU citizens are:
- Medicines are authorized in all EU countries at the same time
- Centralised safety monitoring
- Product Information available in all EU languages at the same time
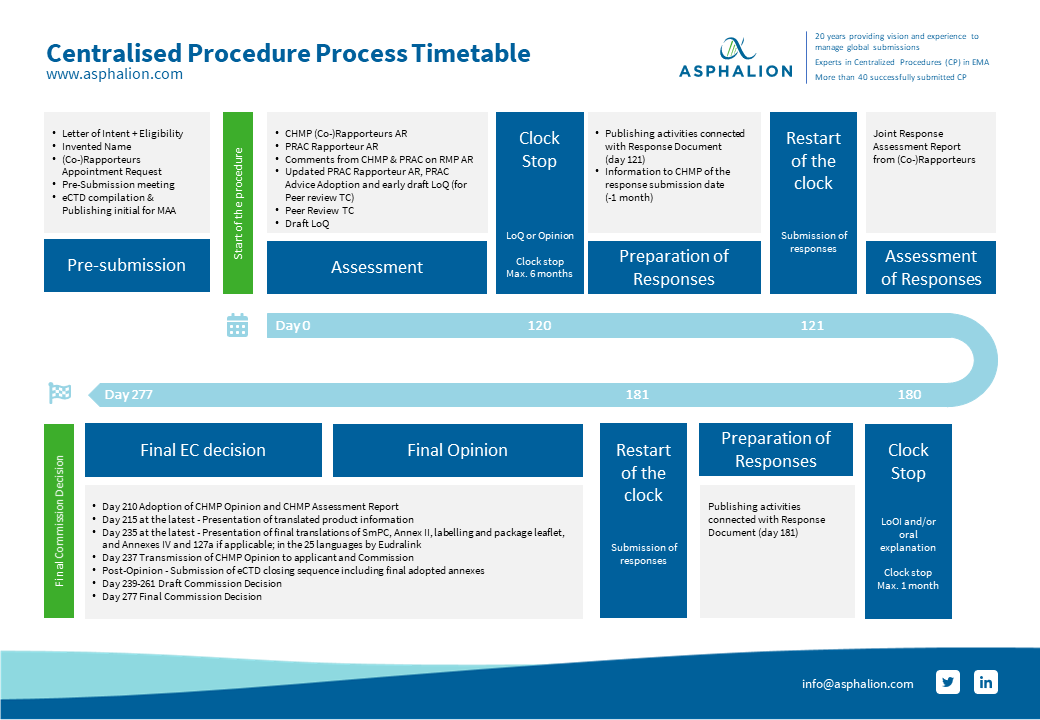
The process of a CP is quite challenging and there are a lot of timelines to be kept, including local activities needed prior to commercialization.
We have summarized all CP steps in this flow chart for you to download and keep always at hand.
Furthermore, you can send us an email: [email protected]
We will be more than happy to assist you!