The centralised procedure (CP) is the European Union-wide procedure for the authorisation of medicines, where there is a single marketing authorisation application to EMA, a single evaluation and a single authorisation. Only certain medicines are eligible for the centralised procedure.
This procedure allows the marketing authorisation holder to market the medicine and make it available to patients and healthcare professionals throughout the EU on the basis of a single marketing authorisation.
Today, the great majority of new, innovative medicines (including COVID-19 vaccines) pass through the centralised authorisation procedure in order to be marketed in the EU.
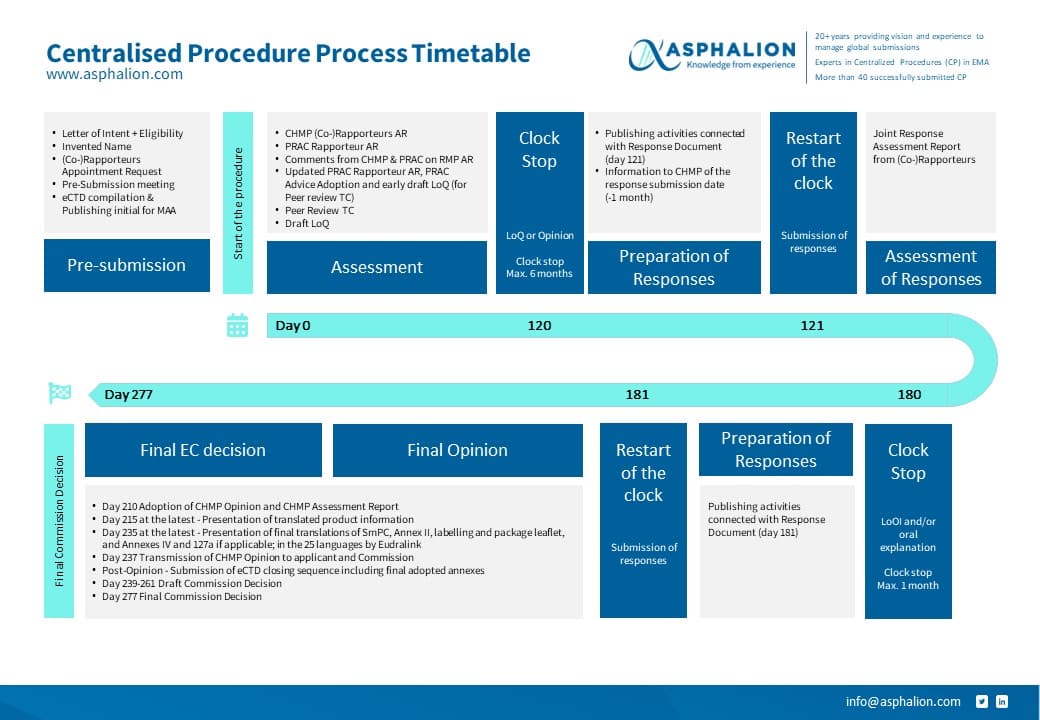
The process of a CP is quite challenging and there are a lot of timelines to be kept, including local activities needed prior to commercialization.
At ASPHALION we are experts in Decentralised Procedures (DCP) in EMA, and have successfully submitted over 40 CP to EMA.
We have summarized all CP steps in this flow chart for you to download and keep always at hand.
Contact us for any further information or requests at: [email protected]