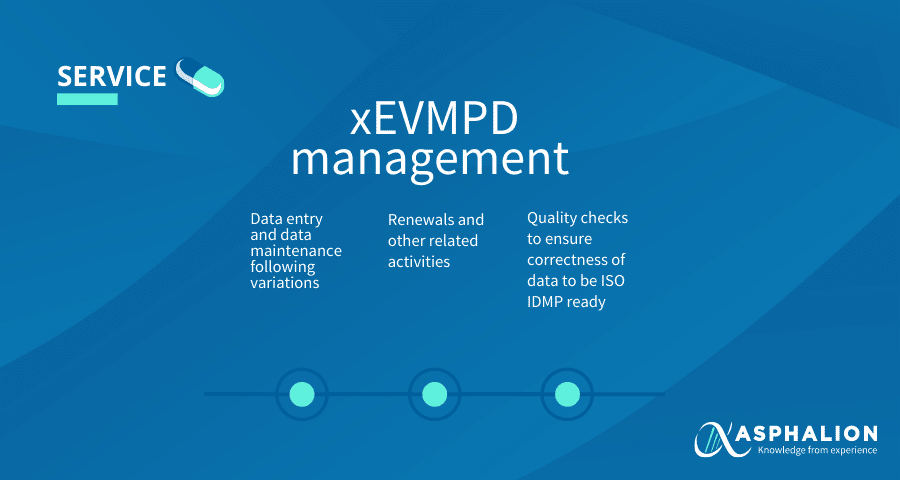
The submission of data on medicines by marketing authorisation holders is a legal requirement from Article 57(2) of Regulation (EC) No. 726/2004, as amended by Regulation (EU) 1235/2010 and Regulation (EU) 1027/2012.
At Asphalion we have a dedicated team, fully trained and greatly experienced in supporting the activities related to xEVMPD. Proactively, we provide pre-filled templates that ease providing the required information for the creation and maintenance of data. We offer a quick and efficient solution that allows you to be compliant and on time.
Check out our #serviceminipill to see how ASPHALION can help you with the xEVMPD management!!
You can check out all pharmaceutical data management services here: DATA MANAGEMENT
For further information, please contact us at: [email protected]