The decentralised procedure (DCP) is the European Union-wide procedure for authorising medicines in more than one European Union Member State in parallel. It can be used for medicines that do not need to be authorised via the centralised procedure and have not already been authorised in any Member State. The procedure falls under the Directive 2004/27/EC.
In the decentralised procedure, the applicant chooses one country as the reference Member State when making its application for marketing authorisation. The marketing authorisation in all chosen Member States is received simultaneously, enabling simultaneous marketing of the medicine and reducing the administrative and regulatory burden.
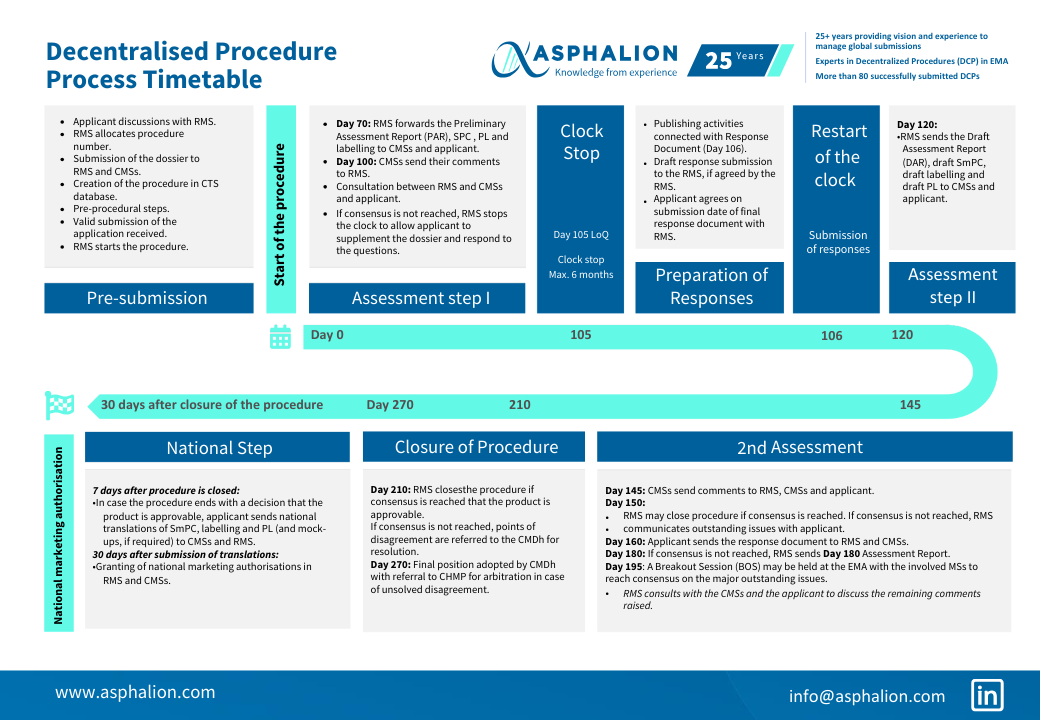
The process of a DCP is quite challenging and there are a lot of timelines to be met.
At ASPHALION we are experts in Decentralised Procedures (DCP), and have successfully submitted over 85 DCP.
We have summarized all DCP steps in this flow chart for you to download and keep always at hand.
Contact us for any further information or requests at: [email protected]