News & Events

WEBINAR | Biological Impurities: Scientific and Regulatory Aspects
Join us for an in-depth session abput “Biological Impurities: Scientific and Regulatory Aspects”, where our Regulatory Affairs

WORLD THALASSAEMIA DAY | RAISING AWARENESS
Today marks International Thalassaemia Day, a global initiative to raise awareness about this rare genetic blood disorder.

EVENT | MedTech Forum 2025
ASPHALION is excited to announce our participation in the MedTech Forum 2025, one of the leading events

NEWS | CHMP Adopts Draft ICH Q1 Guideline on Stability Testing
On April 24, 2025, the Committee for Medicinal Products for Human Use (CHMP) adopted the draft ICH

Optimize your Environmental Risk Assessment (ERA) during the MAA procedure
Ensure compliance. Promote sustainability. Minimize regulatory hurdles.

Asphalion Medical Writing Services | Supporting Your Regulatory Success
Clear, accurate, and regulatory-compliant documentation is essential at every stage of pharmaceutical and biotech product development. At

ARDAT | General Assembly Meeting in Barcelona
Last week, ARDAT project members gathered in Barcelona for the project’s General Assembly. The ARDAT project is
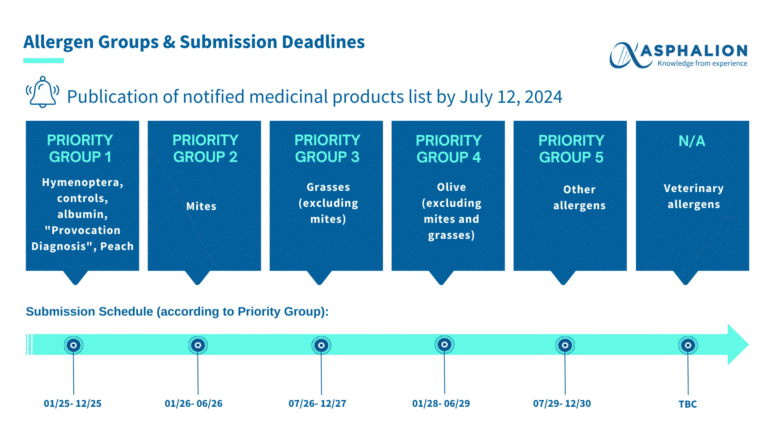
Regulation of Allergen-Based Medicines in Spain | Submission Timelines Reminder
The Spanish Agency of Medicines and Medical Devices (Agencia Española de Medicamentos y Productos Sanitarios) published on
Search or filter



















