We are pleased to announce the publication of the updated EU eCTD Module 1 Specification version 3.1.1, along with the corresponding Validation Criteria version 8.2, by the European Medicines Agency (EMA). These updates introduce several significant changes aimed at improving clarity, standardization, and efficiency in regulatory submissions within the EU.
Key updates include:
- A specific exception now allows the inclusion of tracking tables in submissions to the European Directorate for the Quality of Medicines & HealthCare (EDQM).
- Introduction of a new concept of “super-grouping”, with improved guidance and examples for worksharing procedures, which are now better supported through the EMA’s IRIS case management platform.
- Refined validation rules to differentiate between electronic application forms (eAFs) generated through the PLM Portal and traditional interactive PDF versions. A new validation rule has also been added to ensure that interactive PDF eAFs do not contain attachments.
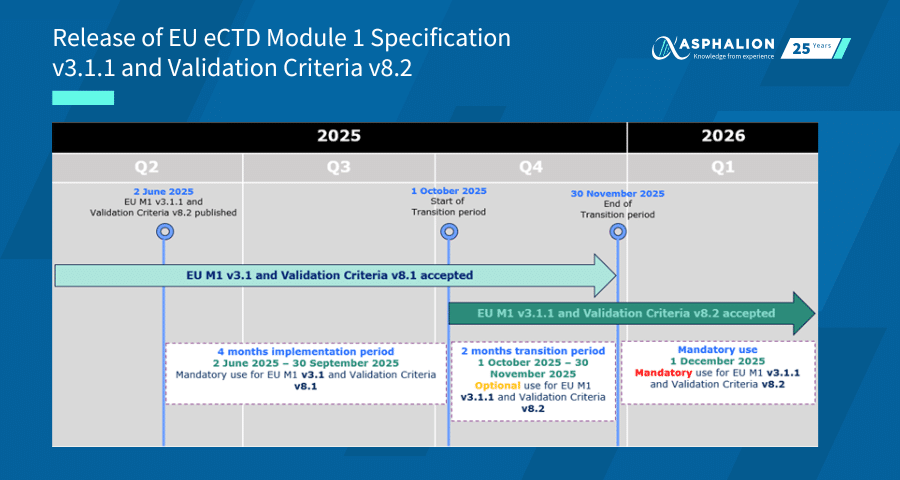
Implementation Timeline:
- From 1 October 2025, both EU M1 versions 3.1 and 3.1.1, along with Validation Criteria versions 8.1 and 8.2, will be accepted by national competent authorities (NCAs) and the EMA.
- From 1 December 2025, only EU M1 version 3.1.1 and Validation Criteria version 8.2 will be accepted. Earlier versions will no longer be permitted.
At Asphalion, we are specialists in regulatory procedures and electronic submissions. Our team has deep expertise in eCTD compilation, validation, and lifecycle management. We are fully aligned with the latest EU requirements and are ready to help you navigate the transition smoothly and efficiently.
If your organization is preparing for this regulatory change, Asphalion can support you in updating your systems, reviewing your submission strategies, and ensuring full compliance with the new standards.
Contact us to learn more about how we can assist with your EU submissions and regulatory operations: [email protected]