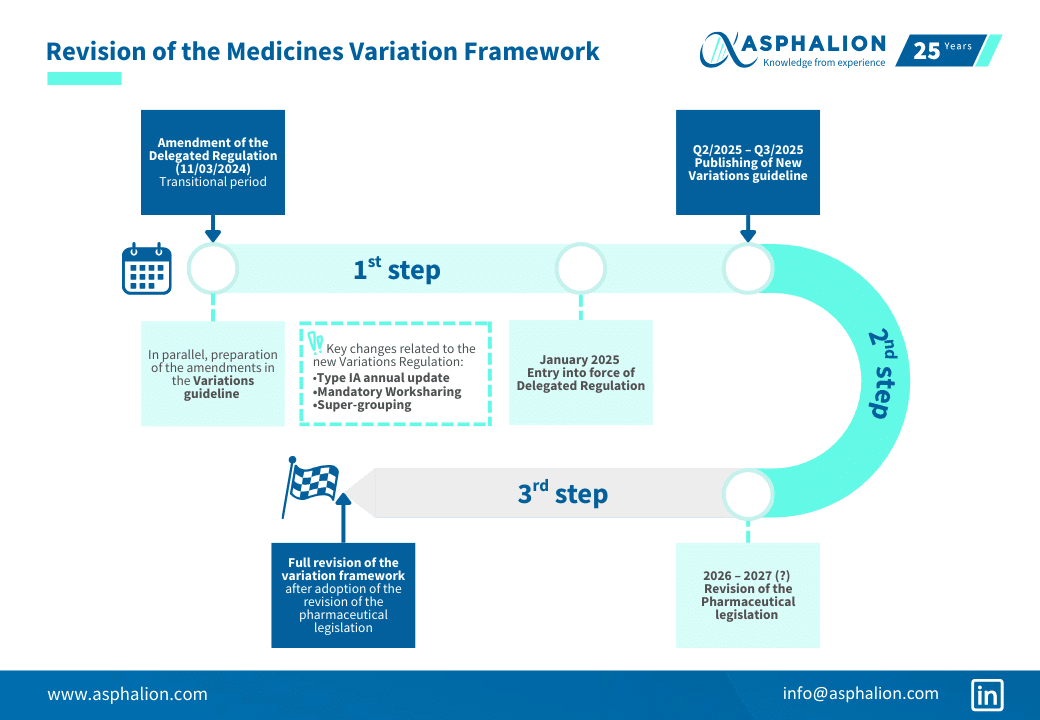
Take a look at this infographic we have prepared to provide a clear overview of the Revision of the Medicines Variation Framework, outlining the key milestones, deadlines, and implementation phases. This visual guide is designed to help stakeholders understand the timeline of changes
At Asphalion, we have many years of proven experience in product Life-Cycle Management, supporting pharmaceutical and biotech companies of all sizes—from large multinationals to small and mid-sized enterprises. We take responsibility for the ongoing maintenance of products across various regional markets, including Spain, the European Union, and the United States. Our services include maintaining and updating technical dossiers and CMC (Chemistry, Manufacturing, and Controls) information to ensure regulatory compliance throughout the product’s life cycle. In addition, we assist with the review of promotional materials to support compliant marketing practices, helping our clients navigate complex regulatory landscapes with confidence and efficiency.
Have a look at the infographic here: Revision of Medicines Variation Framework
Need assistance with your LCM activities? Contact us at: [email protected]