News and Events
Stay in touch with the latest news and upcoming events featuring Asphalion
NEWS | New MDGC Guidance on MDSW
Asphalion is pleased to share the release of the new MDCG guidance on the safe distribution of Medical Device Software (MDSW) apps on online platforms. This guidance provides important insights into the responsibilities of app platform providers under MDR/IVDR and the Digital Services Act. It aims to ensure that software, which supports crucial healthcare functions like insulin pump management and cancer diagnosis, meets required safety and transparency standards. Key highlights include: Regulatory Roles: Defines when app platform providers are considered distributors or importers, subjecting them to MDR and IVDR requirements. Intermediary Services: Clarifies when providers act as intermediaries under the
More News & Events
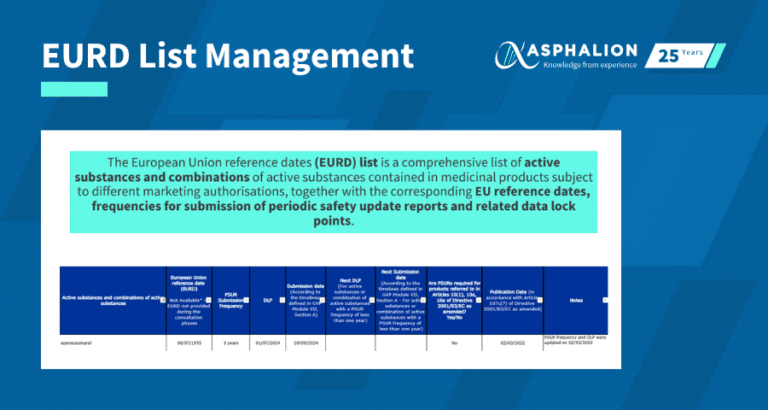
EURD List Management | Stay Compliant and Never Miss a Deadline
Effectively managing the EURD (European Union Reference Date) list is essential for ensuring compliance with pharmacovigilance requirements,

MedTech Week | Medical Devices with Nanomaterials: What You Need to Know Under EU Regulations
Embrace the Power of MedTech this week with Asphalion’s MedTech-Week!

NEWS | New Key Addition to Asphalion
We are excited to announce a significant addition to the Asphalion team!

HORIZON EUROPE | Force Repair 5th Progress Meeting
Last week, the FORCE REPAIR project partners met in Novara, Italy, for the 5th Progress Meeting, hosted
Search or filter