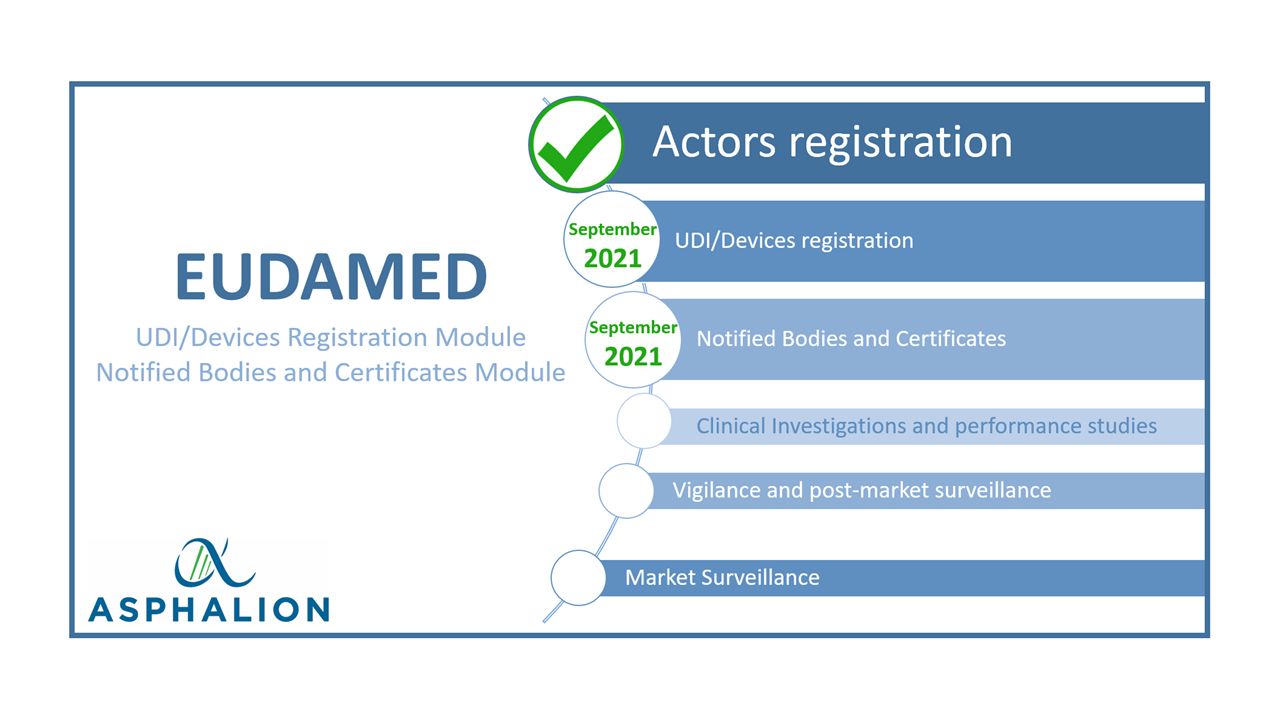
The #EuropeanCommission has stated that the #UDI/device registration module and the #Certificates and #NotifiedBodies module will become available by September 2021.
https://ec.europa.eu/health/md_eudamed/overview_en
For further information do not hesitate to contact us: [email protected]