On May 14th, the International Council for Harmonisation (ICH) published the revised ICH M4Q guide. This guide plays a crucial role in outlining the structure of Chemistry, Manufacturing, and Controls (CMC) information for Modules 2 and 3 of the Common Technical Document (CTD), making it highly relevant for all pharmaceutical submissions.
The revised ICH M4Q guide establishes the groundwork for electronic data standards that facilitate structured applications. It aligns with other ICH guidelines (such as ICH Q8 to Q14) and promotes the adoption of risk-based approaches, including Quality Target Product Profile (QTPP), Technical Risk Assessment (TRA), and Product Control Strategy (PCS).
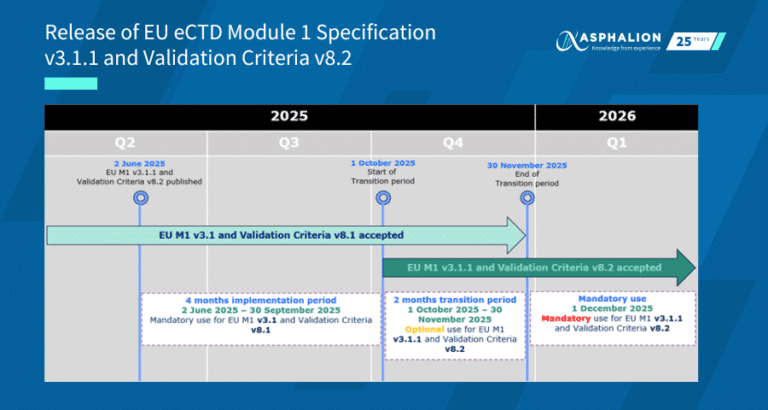
Here’s a timeline of the guide’s key milestones:
- Under public consultation dince May 14th, 2025
- Step 4 is expected by June 2027
- Early adoption is not expected untill 2028
- Implementation will be linked to eCTD 4.0
The implementation of this guide may trigger regional and additional ICH guidelines updates.
We have prepared a SUMMARY WITH THE KEY CHANGES AND UPDATES TO THE GUIDELINE.
If you require any assistance, please contact us at: [email protected]