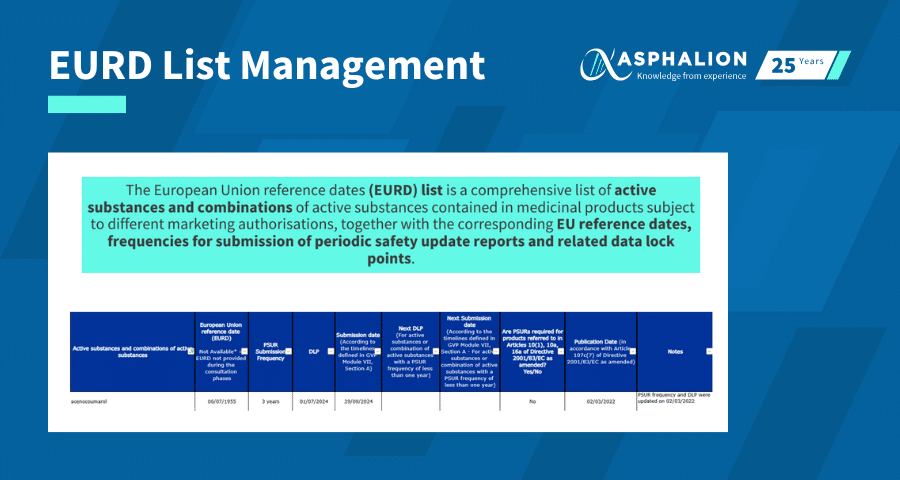
Effectively managing the EURD (European Union Reference Date) list is essential for ensuring compliance with pharmacovigilance requirements, particularly when it comes to submitting Periodic Safety Update Reports (PSURs) on time.
The EURD list is updated regularly by the European Medicines Agency (EMA), and each update may affect the PSUR schedule for one or more of your products. Missing a change can result in non-compliance and regulatory risks. To avoid this, it’s important to implement a robust process for tracking and responding to updates.
Here are some key steps to help you stay organized and compliant:
- Monitor the EMA website regularly
Within your weekly regulatory intelligence activities, make it a habit to check the EMA web page for the latest version of the EURD list, which is typically updated on a monthly basis. - Archive all EURD list versions internally
Keeping a record of all versions helps you track historical changes and provides an audit trail that may be useful for regulatory inspections or internal reviews. - Assess the impact on your product portfolio
Each time a new list is published, review it carefully to determine whether any of your products have been newly included, removed, or had changes to their PSUR frequency or submission deadlines. - Update your internal tracking tools
Maintain an up-to-date Excel sheet or database that includes current PSUR requirements, dates, and any relevant regulatory notes. This ensures that all teams involved are working from the latest information. - Plan ahead and stay organized
Based on the updated data, schedule your PSUR preparation and submission timelines accordingly to avoid last-minute work or missed deadlines.
At Asphalion, we provide end-to-end support in managing EURD list updates and PSUR planning. Our team ensures that you never miss a regulatory change and assists in the efficient preparation and submission of each report, aligned with EMA requirements.
Need support with EURD list tracking or PSUR submissions?
Contact us today and let our regulatory experts help you streamline your pharmacovigilance processes: [email protected]